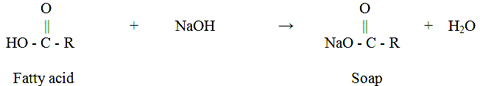Biodiesel is a renewable, biodegradable, non-toxic, low-sulfur new fuel with similar performance to fossil fuel diesel, making it a clean renewable energy alternative to fossil fuels. In the actual biodiesel production process, for every 1 t of biodiesel produced, about 0.1 t of by-product
glycerin is produced. According to the report “Oil World” in Hamburg, the global biodiesel production in 2015 was 29.1 million tons, reaching a record high of 32.8 million tons in 2016, a surge of 11% year-on-year, which resulted in a large amount of by-product
crude glycerin. Therefore, while developing and producing biodiesel, improving the development and utilization of its by-product glycerin will increase the overall utilization and economy of the entire process, and also increase the source of glycerol.
Production of biodiesel by acid, alkali or enzyme catalyzed process,
crude glycerol can be obtained after transesterification, and other glycerin in addition to glycerol, such as water, organic salts, inorganic salts, soap, methanol Or ethanol, pigments and trace amounts of catalysts and glycerides. To apply it to the food, cosmetics and pharmaceutical industries, it is necessary to refine crude glycerin. However, the current crude glycerin refining process is cumbersome, costly, and economically less feasible. Therefore, in order to increase the value of biodiesel by-product glycerin, it is possible to improve the comprehensive utilization value by improving the applicability of crude glycerin in the market and converting high-purity glycerin into high value-added products. Figure 1 shows various products that can be used in the production of biodiesel by-products, crude glycerol and high-purity glycerol. This paper begins with the comprehensive application of biodiesel by-product crude glycerin, and summarizes the current application status of crude glycerin from the fields of chemical products, fuel additives, hydrogen production, fuel cells, methanol or ethanol, and waste treatment. The application prospect of crude glycerin provides technical support for the sustainable development of biodiesel technology.
1 Crude glycerin is used to prepare various chemical products
Using biodiesel by-product crude glycerin as raw material, it can be used to prepare a variety of chemical products, such as 1,2-propanediol, 1,3-propanediol, polyester and polyglycerin. They are important chemical raw materials and products and have a wide range of uses in all aspects.
1.1 1,2-propanediol
1,2-propanediol is an important chemical raw material. Preparation of 1,2-propanediol from crude glycerol is usually carried out by chemical catalytic hydrogenolysis. YUAN et al. used Cu/Mgo as a catalyst to catalyze hydrogenolysis of glycerol to 1,2-propanediol. The preparation method of the catalyst was investigated. It was found that when the catalyst was prepared by coprecipitation, the activity was the highest and the conversion of glycerol was up to The selectivity of 72%, 1,2-propanediol was 97.5%, and the conversion of glycerol was further increased to 82% by the addition of a trace amount of NaOH. CHIU et al [7] used a two-step process to prepare 1,2-propanediol from glycerol. First, glycerol produced intermediate acetol under normal pressure, and then acetol was hydrogenated to form 1,2-propanediol under the action of copper chromate catalyst. The rate reached 75%. Studies have shown that the technology of catalytic hydrogenation of glycerol to bio-based 1,2-propanediol has made great progress, but in order to maintain the high activity of the catalyst, the purity of glycerol is generally required to be high, and further processing and purification of crude glycerol is required. .
1.2 1,3-propanediol
1,3-propanediol is an important intermediate for organic synthesis and an important raw material for the synthesis of polyester PTT (polytrimethylene terephthalate). With the continuous promotion of biodiesel technology, the production of 1,3-propanediol by biological methods using crude glycerol as a raw material has attracted wide attention from researchers all over the world. Several strains for the production of 1,3-propanediol have been reported in the literature, such as Klebsiella pneumoniae, Lactobacillus brevis, Citrobacter freundii, Clostridium butyricum, Pasteur. Clostridium Clostridiumpasteurianum et al. Hu Qiulong et al used the biodiesel by-product glycerol as raw material, Klebsiella as a strain, and fermented 1,3-propanediol to investigate the production efficiency and economic feasibility of different purity glycerol. The experiment found that the refined glycerol (purity > 98) %), crude glycerin A (purity 83%), crude glycerin B (purity 78%) and crude glycerol C (purity 68%) were converted to 1,3-propanediol by 52.38%, 48.08%, 45.22%, respectively. And 39.95%, through the rough evaluation of economic benefits, the crude glycerol A and B production unit 1,3-propanediol cost is lower, while the refined glycerin and crude glycerin C cost is higher. MARIA et al. improved the Clostridium butyricum by metabolic engineering and obtained a recombinant strain DG1. It was found that when glycerol is used as a substrate, 1,3-propanediol can be efficiently fermented and produced at a higher capacity. At a rate of 3g/(L·h), it can be operated continuously for a long time. ANAND et al. used Klebsiellapneumoniae ATCC15380 as a strain, biodiesel by-product crude glycerol as raw material, and fermented to produce 1,3-propanediol. The yield of propylene glycol was 56g/L, and the molar conversion of glycerol was 0.85. The fermentation broth was isolated and purified. A 1,3-propanediol product with a purity of 99.7% is obtained, and as a raw material, a PTT product can be successfully produced to meet the polymerization level requirement.
The production of 1,3-propanediol by biological method has the characteristics of mild reaction conditions, low environmental pollution, and renewable resources. However, its large-scale industrialization still has certain difficulties. The main limiting factors are the high cost of raw materials and the cost of separation and purification process. High, and the production of 1,3-propanediol from crude glycerol is an effective way to reduce the cost of raw materials.
1.3 DHA and PHA
DHA is a kind of ketose which is very beneficial to the human body and is widely used in cosmetics, medicine, food additives and other industries. Using glycerol as a raw material, the production of DHA by microbial metabolism has the characteristics of mild reaction conditions, high raw material utilization rate and high product purity. CHI et al. used crude glycerol as a substrate to ferment DHA with Schizochytrium limacinum microalgae. It was found that crude glycerol can maintain the growth of microalgae and produce DHA. Under the best experimental conditions, the highest yield of DHA is 4.9g. /L, the cell dry weight is 22.1g / L, so the use of crude glycerol as a substrate, microalgae fermentation DHA is a viable solution for the production of DHA. Compared with the chemical preparation of DHA, the microbial process is simple and feasible, easy to control, and is a sustainable development of DHA in the long run.
Polyhydroxyalkanoate (PHA) is a natural polymer biomaterial that has a wide range of applications in medicine, packaging, materials, etc. It is one of the effective ways to use high value-added crude glycerol. ASHBY and other raw materials produced from biodiesel contain glycerin, soap salts and residual fatty acid methyl esters. Pseudomonasoleovorans is used to ferment PHB (polyhydroxybutyrate) and PHA, and biodiesel by-products are found to be coarse. Glycerol can be used to produce PHB and PHA, and the concentration of both products can be controlled by adjusting the growth environment of the strain. KOLLER and other high-permeability microbial fermentation hydrolyzed whey and biodiesel by-product crude glycerol were used as carbon source to produce PHA. It was found that the concentrations of PHA obtained by fermentation of two carbon sources were 5.5g/L and 16.2g/L, respectively. High efficiency in producing PHA. PHA is biodegradable, biocompatible, and has good thermal processing properties. It is a promising polymer, and the biosynthesis of PHA using crude glycerol as one of the raw materials will be one of the important ways to develop and utilize crude glycerol.
1.4 Other chemical products
In industrial microorganisms, glycerin can also be used as a carbon source to produce other valuable chemical products such as succinic acid, propionic acid, citric acid, lactic acid, acrolein, dyes and the like. Among them, acrolein is a multifunctional chemical intermediate that can be used to produce acrylates, superabsorbent polymers and detergents. Acryl aldehyde can be obtained by catalytic dehydration of glycerol by liquid phase or gas phase. The core of the technology is to select a suitable catalyst. OTT and the like use crude glycerol as raw material, subcritical or supercritical water as medium, and obtain acrylic acid by dehydration, but the product yield is not high; the yield of acrylic acid can be improved by adding inorganic acid or inorganic acid salt. In the experiment, The addition of zinc sulfate can achieve a conversion rate of up to 50% at 300-390 ° C and 25-34 MPa. This is mainly because the addition of zinc sulfate reduces the activation energy of the reaction. ZHOU et al. used microporous mesoporous molecular sieve HZSN-5 as a catalyst. The gas phase method catalyzed the dehydration of glycerol to acrolein. The reaction was carried out at 320 ° C. The conversion of glycerol was 98.27% and the selectivity of acrolein was 74.94%. Studies have shown that the use of glycerol to produce acrolein is an active field in the research and application of biodiesel by-product glycerol in recent years, and the core of its technology lies in the selective preparation of catalysts. It can be seen that crude glycerol is used as raw material or substrate, and various chemical products with high added value can be obtained by microbial technology or chemical catalytic hydrogenolysis, oxidation, hydrogenation and the like.
2 Crude glycerin is used to produce hydrogen
Hydrogen is a clean and efficient secondary energy source. With the continuous expansion of hydrogen application and the increasing emphasis on the world's energy and environmental issues, biological hydrogen production technology has received extensive attention. Among them, hydrogen production from crude glycerol is also an important comprehensive utilization of biodiesel by-products, and research in this area has received more and more attention. The main processes for the production of hydrogen from glycerol include steam reforming, partial oxidation, autothermal reforming, aqueous phase reforming and supercritical water reforming. The most widely used in the chemical industry is steam reforming. ADHIKARI et al [17] used hydrogen reforming process to prepare hydrogen, catalyzed high endothermic reaction of glycerol with water to produce hydrogen; investigated the catalytic reforming performance of Ni/MgO, Ni/TiO2 and Ni/CeO2 catalysts, and found Ni /MgO has the highest hydrogen production activity at a reforming temperature of 650 ° C, and the hydrogen yield can reach 56.5%. SLINN et al. investigated the feasibility of hydrogen production from biodiesel by-product glycerol steam reforming. Using Pt-Al2O3 as catalyst, it was found that the higher the reaction temperature, the higher the gas phase yield, the highest yield is close to 100%, and the selectivity is 70%. Under the optimal hydrogen production conditions of glycerol steam reforming, the carbon deposition of biodiesel by-product glycerol is slightly higher than that of pure glycerol, but the catalyst activities of the two are similar. BYRD and other supercritical water reforming processes use biodiesel by-product glycerol as raw material and Au/Al2O3 as catalyst to produce hydrogen. The reaction is carried out in a tubular fixed-bed reactor at a reaction temperature of 700-800 ° C. Glycerol in the feed. The concentration (mass fraction) was 40%, and the highest reaction yield was close to the theoretical yield, and 7 mol of hydrogen per 1 mol of glycerol was obtained. These processes all have certain requirements on the purity of glycerol, because the excess impurities in the crude glycerol will have certain influence on the activity and service life of the catalyst. Therefore, in order to accelerate the efficiency of hydrogen production from crude glycerol and reduce the production cost, it is necessary to develop environmental adaptation. A catalyst with high capacity, corrosion resistance and high activity.
Crude glycerol can also be converted to hydrogen by the form of microbial catalytic conversion. GUILLAUME et al. [20] used photosynthetic bacteria Rhodopseudomonaspalustris to ferment crude glycerol to hydrogen. The yield of this process is high, producing 6 mol of hydrogen per 1 mol of glycerol (75% of the theoretical value, theoretically producing 8 mol of hydrogen per 1 mol of glycerol). The impurities in the biodiesel by-product crude glycerol have no inhibitory or toxic effects on the entire fermentation process. Through the above analysis, using biodiesel by-product crude glycerol as raw material, hydrogen can be produced through various chemical catalytic process technologies or microbial conversion technologies, and the hydrogen production efficiency is high. The process has the advantages of renewable raw materials, cleanliness and no pollution. It is one of the efficient ways to produce hydrogen and has a good space for development.
3 Crude glycerin is used as a fuel additive
Glyceryl alkyl ether is a good fuel additive that improves fuel performance, increases cetane number, increases flow properties, reduces the composition and content of harmful substances in the combustion exhaust, and is used as an additive for diesel and biodiesel. The use of crude glycerin in this technique allows the use of biodiesel by-product glycerin as well as high value-added glyceryl alkyl ether fuel additives.
Among them, glyceryl tert-butyl ether obtained by reacting glycerol with isobutylene or tert-butanol is a promising additive. Adding it to diesel fuel can significantly reduce the content of particulate matter, hydrocarbons and carbon monoxide in the exhaust gas. . KARINEN et al. investigated the etherification reaction of crude glycerol with isobutylene in the liquid phase with acidic ion exchange resin as catalyst. The main reaction of the whole process was etherification reaction. The main products were five ethers and the side reaction was isobutylene. The oligomerization reaction produces C8~C16 hydrocarbons; the molar ratio of isobutylene to glycerol is 3:1, the reaction temperature is 80 °C, the selectivity of the reaction is the best, and the composition of the ether products can be controlled by changing the reaction conditions. And the extent of the etherification reaction. KIATKITTIPONG and other fluidized bed catalytic cracking (FCC) gasoline and glycerol were used as reactants. Amberlyst16, Amberlyst15 and β-molecular sieves were used as catalysts to investigate the effect of etherification reaction on the performance of FCC gasoline. The results showed that compared with the original FCC gasoline. The olefin content of the etherified gasoline product is significantly decreased, and the octane number is increased. When β-molecular sieve and Amberlyst 16 catalyst were used, β-molecular sieve was found to have good catalytic effect, and it is more suitable as a catalyst for etherification reaction.
Glycerol can also be catalytically converted into a fuel additive by acetylation, acetalization, and the like. In its review article, RAHMAT et al. studied the reaction process and specific characteristics of glycerol converted into fuel additives by etherification, acetylation and acetalization, and applied it to gasoline, biodiesel and diesel. The effect of the additives obtained in each reaction. PRADIMA et al. also react different processes of glycerol conversion to biofuel additives (esterification, etherification, acetylation and condensation)