Share the glycerin and glycerin refining equipment related knowledge, need glycerin refining equipment can visit the web:glycerinrefine.com
Wednesday, November 28, 2018
An Innovation in Glycerin Purification
Purification is required to transform crude glycerin to a usable state for existing or emerging uses. The purity requirements for the emerging applications of glycerin vary, and are often intermediate to the crude and refined grades previously established for the classical applications. The salt content in crude glycerin, stemming from the use of homogeneous alkaline catalysts, often ranges from 5 percent to 7 percent, which makes conventional techniques cost intensive. This suggests that for future glycerin markets a new low-cost purification strategy may be more cost-effective than conventional routes .
The glycerin produced in the transesterification of triglycerides reaction is a crude grade. Almost all biodiesel production today involves homogeneous alkaline catalysts such as sodium methylate. The transesterification of triglycerides with methanol generates a methyl-ester phase and a glycerin phase. Impurities such as catalyst, soap, methanol and water are preferentially concentrated in the glycerin phase. The glycerin phase is typically neutralized with acid and the cationic component of the catalyst is incorporated as a salt.
For example, sodium chloride is formed in the neutralization with hydrochloric acid of glycerin containing sodium methylate. Catalyst usage rates vary across the industry, but it is common to find crude glycerin issued from biodiesel production with a salt content of 5 percent to 7 percent. It should be noted that heterogeneous processes using enzyme and solid metal-oxide catalysts are promoted as alternatives to homogeneous alkaline catalysts. Nevertheless, even in heterogeneous transesterification processes, impurities in the natural feedstock materials tend to accumulate in the glycerin phase and therefore, purification may still be required.
Conventional Techniques for Purifying Glycerin
Distillation is the most commonly practiced method for purifying glycerin. The advantages of the distillation process are well known. Namely, it is an established technology that produces high-purity glycerin in high yield. However, the distillation of glycerin is an energy-intensive process . Glycerin has a high heat capacity, which demands a high-energy input for vaporization.
Classical ion-exchange techniques have long been applied to glycerin purification. However, the high salt content of glycerin issued from biodiesel production makes classical ion-exchange uneconomical for this application. Specifically, the chemical regeneration cost for the resins becomes exceedingly high when salt contents approach the 5 percent- to 7 percent-range commonly found in the biodiesel industry.
Tuesday, November 27, 2018
What is liquid glycerin?
Glycerin is the natural form of glycerol. That is, it is mostly glycerol, but has some other components, depending on the source and method of processing. For many commercial uses, this does not make any difference, but it can be purified to essentially pure glycerol for chemical processes.
The fats and oils which are used to make soap are esters of glycerol which are broken down by NaOH to give the sodium salt of the carboxylic acid (soap) and glycerol, which is collected as separate layer. (The different sources of fats and oils will give different impurities.) As both layers are very viscous, separating them is tedious. It is thus amusing to to have soap producers boasting “with added glycerin!” meaning less well separated. “Hand-crafted” soaps typically have most of the glycerin left in, because the backyard producers do not have the technology of the big soap makers, who can produce glycerin of sufficient purity to be sold at a modest price for food and cosmetic use.
Glycerol is also produced when making biodiesel, where methanol CH3OH is used instead of NaOH. Thus it is essentially a low value waste product from two major industrial processes, and produced in far greater amounts than commercial uses demand. Thus many end processes are essentially ways of trying to get some small value from a waste, e.g. by burning it for energy or adding it to cattle feed.
Monday, November 26, 2018
Glycerin's benefits for hair

Glycerin is a carbon compound and humectant which mean it attract the water from the surroundings. In the chemistry it is often called as the glycerol.
Glycerin is widely used in skin and hair products. It is added to soaps, shampoos, moisturizers and lotions. Glycerin is an excellent ingredient in cosmetics due to perfect ability to absorb water from its surroundings. Along with skin, glycerine is also very useful in treating hair.
There are many wonderful benefits of glycerin for your hair:
- Natural moisture retention. Glycerin makes the skin layer improved by making the scalp moisturized. In order to get rid of dehydration the most preferable season to use products with glycerin is winter.
- Maintenance of length. Drying out and breaking out of hair will be reduced with glycerin. Thereby, hair growth will be optimized. It can reach to the adequate length by faster grow.
- Eliminating itch. If you want to get itch free scalp you should apply glycerin over your scalp on a regular basis.
- Reducing dandruff. Glycerin has soothing properties for creating cooling effect on skin layer. That is why dandruff, flaky and dry scalp will be eliminated easily over your skin.
Sunday, November 25, 2018
What is glycerine and how it is used?
Glycerin is a sweet-tasting, neutral, colorless liquid which can turn into a paste when it freezes and has a very high boiling point. While it is flammable, it has a flammability rating of 1 which is very low. It can be easily dissolved in alcohol and water but not in oils. It is an excellent solvent, and many different compounds can be easily dissolved into it.
Glycerin is highly hygroscopic and can easily absorb water from the air. As a result, when 100% glycerin is placed on the tongue, it can result in blisters as it is very dehydrating. However, when it is mixed with water, it can help soften the skin. This is the reason due to which glycerin is so abundantly used in the cosmetics industry.
Moreover, it is also used for manufacturing anesthetics, cough remedies, ear infection drugs and for making capsules. It is also used very commonly for manufacturing soaps, as a freeze control and as an intermediate in the production of chemicals, like polyester polyols. Silver Fern Chemical, Inc is your one-stop destination for buying glycerin and a wide range of other industrial chemicals in bulk quantities. With a well-developed network throughout the US, it is one of the most reputed chemical suppliers in the country.
Friday, November 23, 2018
Crude glycerin distillation
Wednesday, November 21, 2018
Does glycerin help the skin to whiten?

Yes, it helps.
Glycerin is widely used in many beauty products due to its moisturizing and nourishing properties. Glycerin has a wide range of amazing benefits and useful impacts on your skin.
Glycerin lightens the skin complexion and makes it flawless. It not only brings lighter tone for your skin but also protects from tanning. While dealing with skin care, it is recommended to choose natural vegetable glycerin which comes from plant-based oils. Skin will remain beautiful without greasiness. The glycerin is used in majority of the toners in order to make the skin shade lighter.
Glycerin properties:
- Cleaning. In order to get rid of dust and dirt from the skin, you should use glycerin as a cleanser.
- Nourishing. Apply care products with glycerin on your skin daily to keep it supple and soft.
- Great moisturizer. Serving as an excellent moisturizer glycerin reduces dry patches and dull on skin by making your skin absorb water from the air. This makes it especially useful in winter season by safeguarding skin from development cracks. Glycerin hydrates your skin and makes it healthier.
- Glycerin is a brilliant humectant. It is an excellent choice for maintaining water balance. By attracting water from the air glycerin retains water in the skin. Glycerin has excellent hygroscopic characteristics. Water loss is minimized, so, skin’s water balance is optimized on intercellular level. Skin retains hydrated and nurtured.
- Skin protection. Glycerin also protects your skin from damage and saves it from disease-causing bacteria and pollution.
- Gentleness over skin and smoothening. Glycerin is added to skin soaps and lotions as creams because your skin will remain nourished and gentle in this case. It can eradicate the roughness of skin surface and it leaves no harm even over the oily skin.
Tuesday, November 20, 2018
Are glycerine and rose water good for the skin?
The combination glycerin & rose water can actually be extremely beneficial to the skin!
Glycerin is derived from natural fats and oils. It is extremely hygroscopic and has the ability to absorb water.
Its use in cosmetics is very common as it hydrates and moisturises the skin of the face, hair and body.
It is widely used in soap making as a percentage ratio of 15 to 20% or in creams and lotions.
Typical feature about the handmade soaps is that because they contain more glycerin, they are highly softening, especially in combination with vegetable oils such as rose, olive, palm, etc.
Used in hand creams, it protects the skin of the hands from cracking and wounds.
It also strengthens the skin's protective barrier, thus protecting it from adverse weather and air pollution.

Glycerin stimulates the removal of dead skin. After prolonged use of glycerin-containing products, the skin becomes smoother and if you have freckles, they will become lighter.
It also accelerates the healing of the epidermis during injury or skin diseases that are associated with over-keratinization, for example psoriasis.
Rose water has many benefits that you can take advantage of in a variety of ways to improve your skin and overall wellbeing. No matter what your skin type, this 'miracle of nature' will help you look beautiful and radiant! Rose water becomes an essential part of the world of beauty.

It contains vitamins like A and C and has anti-inflammatory properties for natural treatment at home. Rose water can help each skin type, help to soothe sensitive and irritated skin, balance and clean oily skin, rejuvenate, soften, toned, revitalizing and adds beautiful shine to normal skin.
Rose water contains antioxidants and various vitamins that will help to prevent signs of aging and will also feed the skin. Rose water has natural antiseptic and antibacterial properties.
You can use rose water to prevent or reduce the puffiness below the eyes.
Put a bottle full of rose water in the refrigerator for about half an hour and then apply a few drops on a cotton pad. Carefully place it on your eyes. This procedure will help calm the delicate skin around the eyes, while reducing the puffiness under the eyes
Refresh your face by spraying rose water on your skin.
The procedure is very refreshing, especially in the summer. Just spray, several times a day (or you can apply it with a cotton pad). This procedure will instantly refresh the skin, moisturize and nourish it, adding a splendid glow and radiance. You will immediately feel the cooling and moisturizing effect.
Use rose water to soften and nourish dry skin.
Ladies with dry skin can experience the incredible benefits of rose water. You will immediately notice the soothing and softening effect on the skin after applying some rose water on your skin.
Rose water is a natural way to easy remove makeup.
All you have to do is apply the rose water on a cotton pad and gently remove the makeup with massage movements. Rose water also cleans the dust and dirt, it reduces the redness and inflammation of the eyes.
Regular use of rose water can help prevent wrinkles (slows the aging process), shrinks the pores and adds a wonderful shine to the skin.
Use rose water to prevent skin irritation.
After shaving the skin it can become quite irritable. That is why it is highly recommended to apply rose water to soothe it. Not only can it help your skin become softer and nourished, but also, the rose water will leave its amazing and wonderful scent.
Monday, November 19, 2018
Types of Edible Glycerin
Glycerin Absorption and Metabolism
Glycerin is chemically classified as a sugar alcohol, but it is more similar to sugars: it is readily absorbed and is probably converted into glucose in the human body and it provides 4.3 kilocalories of energy per gram [2,3]. Glycerin is not one of the FODMAPs (fermentable oligo-, di- and monosaccharides and polyols), because it is well absorbed in the small intestine and does not pass to the large intestine where it would be fermented by intestinal bacteria.
Glycerin is often mentioned as a sweetener with a low glycemic index, but there are no reliable sources to confirm this.
Types of Edible Glycerin
Vegetable glycerin is made from vegetable oils (palm oil, palm stearin, palm kernel oil, coconut oil, soybean oil) during production of soap or biodiesel.
Animal glycerin is a natural byproduct of animal fats (such as beef tallow) during production of soap.
Synthetic glycerin is produced from cane or corn syrup sugar, or propylene (a petroleum derivative).
Glycerin as a Food Additive
Food-grade glycerin may be added as a humectant (wetting agent), thickener, solvent or sweetener to dairy products (cream), canned goods, confections, fondant, processed fruits, jams, energy bars and other foods. The source of glycerin (animal or vegetable oil, corn syrup, petroleum) used in a food product is usually not revealed on the food labels.
Other Glycerin Uses
- An emulsifier in pills, syrups, toothpastes, mouth washes, fluoride gels, tobacco, etc.
- Anhydrous glycerin is used in fluoride gels, and is approved as an over-the-counter (OTC) anti-caries drug by US Food and Drug Administration (FDA) [14].
- A lubricant, enema or laxative, as a suppository is used to treat constipation.
- Oral glycerin, as a drug, is used to lower high pressure within the eye (glaucoma).
- Intravenous glycerin can be used to treat brain swelling (cerebral edema) [7].
- Glycerin may be used as a skin or hair moisturizer.
Sunday, November 18, 2018
Benefits and Uses of Vegetable Glycerin
1. Cleanser: For this, you will need some glycerin and some citric acid, so you can use either lemon juice or orange juice. Mix it together till it gets to a kind of white, milky texture.

2. Moisturiser: Glycerin works as an amazing moisturiser. Apply it directly on your skin every night, as a night moisturiser, to wake up to hydrated, soft and supple skin.

3. Toner: Mix equal parts of glycerin with rose water in a spray bottle to make the most amazing hydrating toner. Spray this at any time during the day to feel refreshed or when your skin starts to feel dry.

4. Primer: Glycerin can hydrate the skin without leaving any greasy film behind. This is why it can make a perfect base for your makeup. This is one of the best benefits of using glycerin for skin care.

5. Nail Cream: Do you have brittle nails with cuticles that keep showing up because of how dry they are? Then, just use glycerin on your nails to push back the cuticles to where they belong and to make your nails shiny.

6. For Anti-ageing: Mix vitamin E oil from the vitamin E capsules with glycerin to make a perfect serum for dry, ageing skin. Dryness is one of the main reasons for early ageing signs to appear. Ageing skin stops producing as much moisture as younger skin.

7. To Control Oiliness: Mix glycerin with multani mitti to make a face pack that would help get rid of the excessive oil on the face without it being too drying on the face. This is the best way to use glycerin for skin care.
Saturday, November 17, 2018
Research progress of glycerol production methods
At first, glycerol was used only as a skin moisturizer. By 1846, Sobrero reacted glycerol with nitric acid to produce nitroglycerin. Twenty years later, Nobel made nitroglycerin and diatomite into safe explosives, which made nitroglycerin successfully used in the production of Dana explosive. Now, glycerol has been widely used in medicine, cosmetics, alkyd resin, tobacco, food, beverage, polyurethane, celluloid, explosives, textile printing and dyeing, etc. There are about 1700 applications.
Due to the increasing consumption of non-renewable energy such as petroleum, it is the duty of chemical workers to find clean renewable energy. Glycerol, which originates from nature, is non-toxic and harmless, and is an ideal chemical raw material. Therefore, how to develop glycerol well and find its new use has become a research hot spot. This paper reviews the production methods of glycerol, hoping to be helpful to chemical workers who are devoting themselves to developing new uses of glycerol.
Glycerol is widely found in nature in the form of glycerides. Therefore, for a long time, most glycerol is obtained as a by-product in the process of soap production from saponification of oil and fatty acid production from hydrolysis of oil. It was not until 1858 that glycerol could be made from fermentation. During World War I, Germany was the first to create glycerol from sugar beet due to glycerol deficiency. Since 1948, the method of synthesizing glycerol from propylene has been applied in industry. The output has been increasing year by year, and the development trend is fast. At present, according to the source of glycerol, the industrial production methods of glycerol can be divided into three categories: natural glycerol production, fermented glycerol production and synthetic glycerol production. The raw materials of the first 2 methods are renewable.
Friday, November 16, 2018
Metabolism of Glycerin
1.The absorption of glycerin
Glycerin is easily absorbed by human or animal stomach and intestinal mucosa. Animal slices of the small intestine of mice showed that 82% of them were absorbed after 25 minutes if 0.1 mg of glycerol was given. In the same experiment, like gastric slices, the absorption rate of glycerol is slower than that of the small intestine.Experiment on slicing of hamster intestine, the absorption rate of glycerol was 25% of glucose.
Experiments on human body: Oral glycerin 5g, when 15min, the highest concentration of glycerol in serum. For adults, it is 0.46-1.85mg/dL with a turnover speed of 550g/h. From the animal experiment of labeling glycerin with 14c, the glycerin in blood decreased rapidly after 4H of intravenous injection.
In addition, the absorbed glycerin is distributed in the brain and liver. Kidney and other body parts. Glycerin is hardly absorbed by the skin.
2.Glycerin Excretion
14% of glycerin is consumed by rabbits and rats. After 48H, the tendency of glycerol was observed: 65% of glycerol was converted to carbon dioxide and excreted by absorption, 8.4% of glycerol was excreted in urine and the rest was excreted in feces.
For the human body, if a large amount of glycerol is taken, it will not metabolize and excret directly in the urine. When taking 8.9-17.8G, there is no urinary excretion. When taking 20G, there are traces of excretion. When taking 26G, 0.5-0.1G was detected and 200G was excreted in total 200G. The excretion of urine increased by 40%-50% compared with that of oral administration.
3.Glycerol Metabolism
Glycerol absorbed in the body is first converted to glycerol -3- phospholipid by the action of glycerol activating enzyme. Glycerol induced enzymes are distributed in liver, kidney, heart or mammary gland. Glycerol -3- phospholipid is the intermediate product of sugar metabolism.
- Glycerol-3-phospholipid has the same metabolic effect as sugar. It is decomposed into water and carbon dioxide through TCA cycle, or vice versa to produce glucose and glycogen for new glucose metabolism. It has been reported that 75% of glycerol in serum changes to glucose.
The metabolism of glycerin in the body becomes the source of energy supply, and the heat generated is 4.32Kcal/g
(2) glycerol -3- phosphoric acid was converted to monoglyceride, Diglycerin, three glyceride and phospholipid.
(3)After transporting glycerol-3-phosphate from cytoplasm to glandular granules, glycerol-3-phosphate dehydrogenated to dihydroxyketone phosphate due to the action of enzymes, FADH was produced at this time, but FADH was utilized as an electron donor of oxidative phosphorylation in glandular granules, which is a well-studied and proven way.
Glycerol in the body can increase insulin, but unlike hexose such as glucose, the effect of insulin is not affected. In addition, glycerol has the effect of preventing ketone formation and preventing the production of acetic acid and other ketone bodies. In addition, the role of glycogen amino acids in the production of new sugars is also prevented.
Thursday, November 15, 2018
Where does glycerin come from?
In 1889, a viable way to separate the glycerin out of the soap was finally implemented. Since the number one use of glycerin was to make nitroglycerin, which was used to make dynamite, making soap suddenly became a lot more profitable! I have an untested theory that you could trace the roots of most big soap-makers (and the “fall” of the small, local soap-maker) to about this time in history.
The process of removing the glycerin from the soap is fairly complicated (and of course, there are a lot of variations on the theme). In the most simplest terms: you make soap out of fats and lye. The fats already contain glycerin as part of their chemical makeup (both animal and vegetable fats contain from 7% – 13% glycerine). When the fats and lye interact, soap is formed, and the glycerin is left out as a “byproduct”. But, while it’s chemically separate, it’s still blended into the soap mix.
While a cold process soap-maker would simply pour into the molds at this stage, a commercial soap-maker will add salt. The salt causes the soap to curdle and float to the top. After skimming off the soap, they are left with glycerin (and lots of “impurities” like partially dissolved soap, extra salt, etc.). They then separate the glycerin out by distilling it. Finally, they de-colorize the glycerin by filtering it through charcoal, or by using some other bleaching method.
Glycerin has lots of uses besides being used to make nitroglycerin (note: glycerin is not an explosive substance by itself. It has to be turned into nitroglycerin before it becomes explosive, so it’s safe to work with in your kitchen). Some uses for glycerin include: conserving preserved fruit, as a base for lotions, to prevent freezing in hydraulic jacks, to lubricate molds, in some printing inks, in cake and candy making, and (because it has an antiseptic quality) sometimes to preserve scientific specimens in jars in your high school biology lab.
Glycerin is also used to make clear soaps. Highly glycerinated clear soaps contain about 15% – 20% pure glycerin. Known as “Melt and Pour” soaps, these soaps are very easy for the hobbyist to work with. They melt at about 160 degrees Fahrenheit, and solidify fairly rapidly. Because of their high glycerin content, the soaps are very moisturizing to the skin. Unfortunately, this high glycerin content also means that the soaps will dissolve more rapidly in water than soaps with less glycerin, and that if the bar of soap is left exposed to air, it will attract moisture and “glisten” with beads of ambient moisture.
These downsides, however are more than compensated by the emollient, skin loving and gentle nature of this soap which is especially good for tender skin and children.
(1) The pure chemical product is called Glycerol (which shows that it is an alcohol), while the impure commercial product is called Glycerin. This is a technical complexity, so for this article, I’m sticking to the more familiar term, Glycerin.
Wednesday, November 14, 2018
Glycerin in hair cosmetics
Glycerin well moisturizes and helps retain moisture in the hair, which is very useful for dry and curly hair and skin. It is very hygroscopic and in a concentrated form it can draw water from the skin to form bubbles. In diluted form, as it is used in cosmetics, glycerin for hair and skin is completely safe.
When using glycerin for hair, you need to remember that as one of the water-holding substances, it is very sensitive to environmental humidity. In very dry weather, cosmetics with a high content of glycerin can cause dry hair, and in high humidity, on the contrary, it is very moisturized, which is especially bad for very curly hair. But if it is combined in composition with oils and water, then such a product will be effective regardless of the climate and time of year.
Applying glycerin on the hair you need to remember that it conducts heat well, especially in comparison with silicones, proteins and polyquaterniums. Therefore, if protection against temperature is necessary, glycerin for hair is used in small quantities together with insulating and protective moisturizing components (thermal protection).
Since glycerin dissolves many substances well, it is worthwhile to be cautious to people who dye their hair with seven-permanent dyes, as it can quickly wash the color from the hair. Similarly, glycerin will contribute to the fading of the color of dyed hair with permanent and demi-permanent paint. By itself, this component does not lighten the hair, however, if there was a fresh dyeing, it contributes to the leaching of color from the hair, similarly to a deep cleaning shampoo. After 48 hours after dyeing, when the color is fully entrenched on the hair, the use of glycerin will no longer affect the leaching of color. But up to this point on the use of care with glycerin should be abandoned.
Often used glycerin in the composition of shampoos, where by passing the solvent properties helps to lower the freezing point of the finished product. Indeed, shampoos, like many other products for hair and skin, contain large amounts of water that freezes at 0 ° C. This temperature is often in winter in our climate and can be a problem. Since, as it freezes, the product can not only deteriorate, but break the capacity in which it is located. This would create huge problems when transporting cosmetics in the winter, but this is not the case, and modern cosmetics can withstand fairly low temperatures. This is due to glycerol and similar products that lower the freezing point and make them stable in the winter.
Tuesday, November 13, 2018
The main properties of glycerin and use
Glycerin is very hygroscopic and easily adsorbs water from the air. If the vial of pure glycerin is left open, it will absorb a significant amount of water from the air.
Despite the fact that glycerin is a by-product in the manufacture of soap, in its composition it can be found in large quantities only in expensive varieties of soap, including special glycerin soap, where it helps keep moisture on the skin, as well as improves cleansing properties soap. Cosmetic formulations with 20-25% glycerol are used to treat dry skin. It is worth noting that even the use of 20% glycerol solutions on dry, sensitive skin showed no signs of irritation, therefore, glycerin soaps are often used by people with sensitive skin.
Glycerin can dissolve a lot of oils and herbal essences. Because it can often be found in cosmetics for hair and skin in small quantities, as a solvent. Also, this component is a good dispersing agent for pigments and is widely used in makeup cosmetics.
In the formulations of cosmetics, its amount is limited, since an excess of glycerol can lead to the stickiness of creams, lotions and other cosmetics. Therefore, it is used in seven with other substances of similar properties, for example, sorbitol.
Glycerin is widely used in cosmetics for hair, especially it is often used to care for dry, brittle and curly hair. As an effective moisture-retaining agent glycerin applied to moisturized hair, prevents their excessive drying. Hair after its application is soft and elastic. No less effective, it helps fill dry scalp with moisture. When using drugs with a high content of glycerin on oily scalp and hair prone to fat, it can exacerbate the effect of "greasy" hair.
Glycerin, like many other water-retaining components, may not be controlled when used in very dry or very humid climates. In very dry weather, it can absorb moisture from skin and hair and dry them too much. While in very wet weather, it will unnecessarily moisturize the hair, leading to excessive fluffiness. For this reason, in the formulations of cosmetic preparations, glycerin is usually combined with other moisturizing and emollient substances, including oils. This helps avoid unwanted effects.
Glycerin (Glycerin) in cosmetics helps to reduce the viscosity of the final product, as well as reduce the freezing temperature. This allows you to transport cosmetics even in the winter at low temperatures, without fear of freezing. This is especially true of cosmetic products with a high water content: shampoos , lotions.
Sunday, November 11, 2018
Where does glycerin come from?
In 1889, a viable way to separate the glycerin out of the soap was finally implemented. Since the number one use of glycerin was to make nitroglycerin, which was used to make dynamite, making soap suddenly became a lot more profitable! I have an untested theory that you could trace the roots of most big soap-makers (and the “fall” of the small, local soap-maker) to about this time in history.
The process of removing the glycerin from the soap is fairly complicated (and of course, there are a lot of variations on the theme). In the most simplest terms: you make soap out of fats and lye. The fats already contain glycerin as part of their chemical makeup (both animal and vegetable fats contain from 7% – 13% glycerine). When the fats and lye interact, soap is formed, and the glycerin is left out as a “byproduct”. But, while it’s chemically separate, it’s still blended into the soap mix.
While a cold process soap-maker would simply pour into the molds at this stage, a commercial soap-maker will add salt. The salt causes the soap to curdle and float to the top. After skimming off the soap, they are left with glycerin (and lots of “impurities” like partially dissolved soap, extra salt, etc.). They then separate the glycerin out by distilling it. Finally, they de-colorize the glycerin by filtering it through charcoal, or by using some other bleaching method.
Glycerin has lots of uses besides being used to make nitroglycerin (note: glycerin is not an explosive substance by itself. It has to be turned into nitroglycerin before it becomes explosive, so it’s safe to work with in your kitchen). Some uses for glycerin include: conserving preserved fruit, as a base for lotions, to prevent freezing in hydraulic jacks, to lubricate molds, in some printing inks, in cake and candy making, and (because it has an antiseptic quality) sometimes to preserve scientific specimens in jars in your high school biology lab.
Glycerin is also used to make clear soaps. Highly glycerinated clear soaps contain about 15% – 20% pure glycerin. Known as “Melt and Pour” soaps, these soaps are very easy for the hobbyist to work with. They melt at about 160 degrees Fahrenheit, and solidify fairly rapidly. Because of their high glycerin content, the soaps are very moisturizing to the skin. Unfortunately, this high glycerin content also means that the soaps will dissolve more rapidly in water than soaps with less glycerin, and that if the bar of soap is left exposed to air, it will attract moisture and “glisten” with beads of ambient moisture.
These downsides, however are more than compensated by the emollient, skin loving and gentle nature of this soap which is especially good for tender skin and children.
(1) The pure chemical product is called Glycerol (which shows that it is an alcohol), while the impure commercial product is called Glycerin. This is a technical complexity, so for this article, I’m sticking to the more familiar term, Glycerin.
Saturday, November 10, 2018
Purification of sweet water
- Purification of Sweet Water without Catalyst
The quality of sweet water without splitting decomposition of oil depends on the quality of the oil. After degumming and alkali refining, there are few impurities in the sweet water after pyrolysis, and the concentration of glycerol is between 10% and 25%. The purification method is also simple.
Purification operation method: Now the sweet water is heated to about 70 degrees Celsius, static settlement as far as possible, the upper layer of fat is skimmed out. If the sweet water is opaque (especially in medium temperature and medium pressure hydrolysis operation, when the hydrolysis depth is low, or when the high pressure hydrolysis interface is controlled low), it shows that there are more incomplete hydrolysis of fat mixed, inorganic acid or salt can be added a little to destroy its emulsification. Separation of fat. Otherwise, the effect of sweet water treatment is not good, which not only affects the evaporation of purified water, but also makes the filtration difficult.
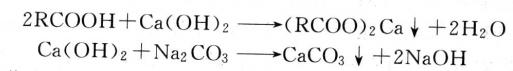
At 60-70℃, Na2CO3 solution was added to the compressed air, and the PH value was controlled at about 9. CaCO3 precipitation was formed and precipitated. The purified water was purified after filtration.
When refined glycerol is not produced by distillation, the second clean water can be decolorized by activated carbon adsorption, and then purified by ion exchange resin to obtain pure purified sweet water. The refined glycerol with more than 98% glycerol content can be obtained by direct evaporation and concentration of purified sweet water treated by ion exchange resin.
Compared with distilled glycerol, refined glycerol without distillation has poor thermal stability and colour, but the recovery of glycerol is greatly improved. It is necessary to study carefully whether the low quality sweet water with more impurities is treated with ion exchange resin.
Protein and other impurities in sweet water are easy to ferment and deteriorate. They are not suitable for long-term storage. Sweet water should be purified and treated in time. In order to reduce glycerol loss, the sweet water container can be cleaned regularly.
Aluminum salts have a good effect on fatty acid treatment, and the adsorptive effect of the formed fatty acid aluminium salts is also good. Aluminum salts are mostly used abroad to treat fatty substances in sweet water, and quicklime is mostly used in China. If conditions permit, aluminium salts are also worth considering.
Friday, November 9, 2018
Production method of glycerol
Before 1984, glycerol was recovered from by-products of soap making from animal fat or vegetable oil.Until now, natural oils and fats are still the main raw materials for the glycerol production. About 42% natural glycerol is produced from the by-product of soap making and 58% by fatty acid production.Saponification of oils and fats in soap making industry,the product of saponification reaction is divided into two layers: the upper layer mainly contains salt (soap) and a small amount of glycerol, the lower layer is waste lye, a dilute glycerol solution containing salt, sodium hydroxide, generally containing 9-16% glycerol, inorganic salt 8-20%.In recent years, the continuous high pressure hydrolysis method has been widely used, the reaction does not use catalyst, the purification method is simpler than the waste alkali solution.The glycerol content in the waste liquor of soap making or the glycerol water obtained by hydrolysis of oil and fat is not high, and it contains various impurities. The production process of natural glycerol includes purification, concentration to crude glycerol, and distillation, decolorization and deodorization of crude glycerol.
Synthetic glycerol
Many ways to synthesize glycerol from propylene can be classified into two main categories, chlorination and oxidation. Acrylonitrile and propylene oxidation are still used in industry.
Industrial grade glycerin
Industrial grade glycerol is diluted with 1/2 star distilled water, stirred sufficiently, activated carbon is added, and heated to 60~70 ℃for decolorization.Then vacuum filtration is used to ensure that the filtrate is clear and transparent.The filtrate was added to the pre-treated 732 strong acid positive resin and 717 strong alkali negative and negative resin column to remove electrolytes and impurities in glycerol by controlling the dropping acceleration.After removing impurities, the glycerol solution is distilled by vacuum distillation, and the vacuum degree is more than 93326Pa. The temperature of the kettle is between 106 and 108 ℃. After evaporating most of the water, the kettle temperature is raised to 120 ℃ for rapid dehydration. When the kettle is not in water, it stops heating. The materials in the kettle are finished products, and are used as automobile and aircraft fuel and antifreeze in oil field.
Wednesday, November 7, 2018
Difference Between Triglycerides & Phospholipids

The bodies of all living things have cells. However, cells cannot function properly without the presence of certain substances, such as lipids. Lipids are a group of naturally occurring molecules that include animal fats, vegetable fats, certain vitamins, triglycerides and phospholipids. At first glance, triglycerides and phospholipids appear very similar. But they have slightly different chemical structures and serve differing functions.
TL;DR (Too Long; Didn't Read)
Triglycerides and phospholipids are both lipids that serve certain functions in the body. However, they differ slightly in structure and function. Triglycerides have glycerol and three fatty acids, which makes them fats. Phospholipids are not fats, since they have glycerol, two fatty acids and phosphorus. Phospholipids are more essential to the formation of lipid bilayers, which maintain cell membrane structure, than triglycerides are. Fat cells store triglycerides, while phospholipids help break down fats in the body.
Structure and Functions of Triglycerides
Triglycerides are a kind of fat found in the bodies of both plants and animals. In plants, triglycerides appear in oils such as peanut oil, while in animals triglycerides live in fat cells. In both plants and animals, triglycerides share the same structure. A single triglyceride molecule has glycerol and three fatty acids.
Triglycerides serve several functions in the body. First, they help maintain the structure of cell membranes by forming a lipid bilayer. This helps keep the inside and outside of cells separate, so organelles cannot drift out of the cell, and foreign substances cannot get in, except under special circumstances.
Tuesday, November 6, 2018
What Are the Functions of Triglyceride Phospholipid & Sterol?

Lipids are organic compounds (that is, they contain carbon, hydrogen and oxygen) that are not water-soluble, instead dissolving in fatty solvents. Three types of lipids are found in the human body and in the foods that people eat: triglycerides, phospholipids and sterols. "Lipid," "fat" and "oil" are often used interchangeably in a nutrition context; solid lipids make up fats, while lipids in liquid form are called oils.
Just as nucleotides are the basic units of DNA molecules, fatty acids are basic units of structure in triglycerides and phospholipids. The basic structural unit of a sterol is a group of four connected carbon-hydrogen rings.
Triglyceride Structure and Function
Triglycerides consist of a glycerol "backbone" along with three fatty acids bonded to the backbone in an ester linkage. Glycerol is a three-carbon molecule, C(H2)OH-C(H)OH-C(H2)OH. When one of its hydroxyl groups (-OH) loses a hydrogen, a fatty acid can bind to the oxygen in its place, creating a C-O-C (ester) bond. Fatty acids are four to 24 carbons long; if they have even one double bond, they are considered unsaturated, but are otherwise classified as saturated.
Triglycerides are overwhelmingly the predominant type of lipid found in nature, accounting for 99 percent of lipids in the body and 95 percent of dietary lipids. Triglycerides function in the body mainly as fuels, supplying 9 calories of energy per gram.
The importance of triglycerides in health is undisputed. An overly high triglycerides level is a risk factor for heart disease. On the other hand, some fatty acids are essential, meaning that the body cannot make them and must be ingested from foods. One of these is the omega-3 triglyceride linolenic acid.
Phospholipid Structure and Function
Phospholipids are fat-related molecules that include phosphorus, fatty acids and a nitrogen-containing base. Like triglycerides, they have a glycerol backbone, but it is attached to two fatty acids and a phosphorus group rather than three fatty acids.
Phospholipids are essential to cells because they make up most of the cell membrane. The phospholipid lecithin is used as an emulsifier in food products, meaning that it keeps fats and liquids mixed together, as in salad dressings. They are also found in wheat germ, peanuts, egg yolks, soybeans and organ meats such as liver.
Sterol Structure and Function
Sterols are made up primarily of a signature four-ring structure that contains carbon and hydrogen atoms. Cholesterol is the best known sterol, which is vital in cell membrane structure and is the foundation of numerous important compounds in the body. It is found only in foods of animal origin, but humans do not need to ingest any cholesterol because the body can make what it needs.
Sterols are waxy substances to the touch and do not readily dissolve in water. Some plant sterols can block the absorption of dietary cholesterol.
Extra: Fatty Acid Basics
Saturated fatty acids are solid, whereas unsaturated fatty acids are liquid. Dietary fats consist of both saturated and unsaturated fatty acids. A fatty acid with one double bond is called monounsaturated, and those with two or more are called polyunsaturated. Fatty acids provide immediate energy and can be efficiently stored for later use. They also provide insulation, protection and, in some cases, satiety, and they transport fat soluble vitamins.
Monday, November 5, 2018
How to Make Glycerin From Vegetable Oil

Glycerin, or glycerol, is a colorless and odorless syrup that tastes sweet and is a byproduct of saponification – the process of making soap – of natural fats, such as vegetable oil. You can make glycerin yourself using heat and some lye, which can then be used to make things like soap or skin moisturizer.
Protect Yourself First
Take precautionary measures by protecting yourself from the harmful chemicals you will be subjected to such as the lye, which is corrosive. Wear safety goggles through the entire process. and wear gloves when dealing with high temperatures and chemicals like lye.
Making Glycerin
1. Measure 4 tsp. of lye and pour it into a pot. Add into the pot 2 cups of vegetable oil along with 1 cup of water. You can buy lye from companies that deal with soap ingredients or make it yourself at home from wood ash and water.
2. Begin heating the mixture and place a thermometer into the pot as you stir frequently. Continue heating the mixture for 20 minutes, until the reading on the thermometer is 125 degrees Fahrenheit. Reduce the heat until the temperature drops to 100 degrees Fahrenheit.
3. Soak the mixture at this temperature (125 degrees Fahrenheit) and stir for about 10 to 15 minutes. Remove the pot from the heat source after the mixture thickens and add 4 tsp. of salt while still hot.
4. Leave the mixture to cool while still and you should be able to observe soap forming at the top and glycerin at the bottom. Soap cannot dissolve in glycerin and that’s why they appear as so. Separate the mixture by simply poring off the soap or skimming it off if you are not planning to use it again. You may mold glycerin in a desirable shape by using the soap mold then freezing it.
Sunday, November 4, 2018
Value-added uses for crude glycerol
The dramatic increase in demand for transportation fuels and the increase in environmental concerns, coupled with diminishing crude oil reserves, have increased the emphasis on renewable energy. Biodiesel, one of the promising alternative and renewable fuels, has been viewed with increasing interest and its production capacity has been well developed in recent years. Although world biodiesel production was expected to reach a high capacity, in fact, it is less than the anticipated target and has increased at a slower rate . The main reason is its relatively high production cost. Utilization of the glycerol co-product is one of the promising options for lowering the production cost.
Biodiesel production will generate about 10% (w/w) glycerol as the main byproduct. In other words, every gallon of biodiesel produced generates approximately 1.05 pounds of glycerol. This indicates a 30-million-gallon-per-year plant will generate about 11,500 tonnes of 99.9 percent pure glycerin. It was projected that the world biodiesel market would reach 37 billion gallons by 2016, which implied that approximately 4 billion gallons of crude glycerol would be produced . Too much surplus of crude glycerol from biodiesel production will impact the refined glycerol market. For example, in 2007, the refined glycerol's price was painfully low, approximately $0.30 per pound (compared to $0.70 before the expansion of biodiesel production) in the United States. Accordingly, the price of crude glycerol decreased from about $0.25 per pound to $0.05 per pound . Therefore, development of sustainable processes for utilizing this organic raw material is imperative.
Since purified glycerol is a high-value and commercial chemical with thousands of uses, the crude glycerol presents great opportunities for new applications. For that reason, more attention is being paid to the utilization of crude glycerol from biodiesel production in order to defray the production cost of biodiesel and to promote biodiesel industrialization on a large scale. Although intensive investigations have focused on utilizing crude glycerol directly, review papers on crude glycerol utilization are scarce. This review mainly addresses the current and potential value-added applications of crude glycerol from biodiesel production.
Chemical compositions of crude glycerol
The chemical composition of crude glycerol mainly varies with the type of catalyst used to produce biodiesel, the transesterification efficiency, recovery efficiency of the biodiesel, other impurities in the feedstock, and whether the methanol and catalysts were recovered. All of these considerations contribute to the composition of the crude glycerol fraction. For instance, Hansen et al. studied the chemical compositions of 11 crude glycerol collected from 7 Australian biodiesel producers and indicated that the glycerol content ranged between 38% and 96%, with some samples including more than 14% methanol and 29% ash. Such variations would be expected with small conversion facilities. In most cases, biodiesel production involves the use of methanol and a homogeneous alkaline catalyst, such as sodium methoxide and potassium hydroxide. Accordingly, methanol, soap, catalysts, salts, non-glycerol organic matter, and water impurities usually are contained in the crude glycerol. For example, crude glycerol from sunflower oil biodiesel production had the following composition (w/w): 30% glycerol, 50% methanol, 13% soap, 2% moisture, approximately 2-3% salts (primarily sodium and potassium), and 2-3% other impurities . Moreover, while the same feedstocks were employed, the crude glycerol from alkali- and lipase-catalyzed transesterifications contained different purities of glycerol . The salt content in crude glycerol, from biodiesel production via homogeneous alkaline catalysts, ranged from 5% to 7% which makes the conventional purification techniques more costly. Heterogeneous processes using enzymes and solid metal-oxide catalysts have been promoted as good alternatives to homogeneous alkaline catalysts in terms of improving the quality of crude glycerol. However, even in heterogeneous transesterification processes, impurities existing in the natural raw feedstocks tend to accumulate in the glycerol phase. Therefore, purification of crude glycerol is required, in most cases, to remove impurities in order to meet the requirements of existing and emerging uses.
Value-added opportunities for crude glycerol
Worldwide, crude glycerol derived from biodiesel conversion has increased from 200, 000 tonnes in 2004 to 1.224 million tonnes in 2008 . Meanwhile, the global market for refined glycerol was estimated to be roughly 900, 000 tonnes in 2005 . Therefore, it is of great importance for scientists to find new applications for refined and crude glycerol. Recently, numerous papers have been published on direct utilization of crude glycerol from biodiesel production. In the following sections, detailed discussions on utilization of crude glycerol are presented.
Animal feedstuff
Using glycerol as a feed ingredient for animals dates back to the 1970s . However, glycerol's utilization in feeds has been limited by the availability of glycerol . Recently, the possibilities of using crude glycerol from biodiesel in feeds have been investigated because of the increase in the price of corn and the surplus of crude glycerol.
Crude glycerol in non-ruminant diets
Glycerol has high absorption rates and is good energy source. Once absorbed, it can be converted to glucose for energy production in the liver of animals by the enzyme glycerol kinase . Crude glycerol samples, from different biodiesel producers, were analyzed as energy sources. The digestible energy (DE) values for 85% of the crude glycerol samples were in the range of 14.9-15.3 MJ/kg with metabolizable energy (ME) values in the range of 13.9-14.7 MJ/kg . Crude glycerol was an excellent source of calories for non-ruminants, for example, the ME determined in broilers, laying hens and swine were 15.2, 15.9 and 13.4 MJ ME/kg, respectively . In growing pigs and laying hens, 14.0 MJ/kg apparent DE and 15.9 MJ/kg nitrogen-corrected apparent ME (AMEn) were reported, respectively, which implied that crude glycerol was used efficiently. The AMEn of crude glycerol was metabolized efficiently by broiler chickens with an AMEn of 14.4 MJ/kg. That was very similar to the general energy (GE) of 15.2 MJ/kg . In nursery pigs, the GE concentration of crude glycerol depended on the concentration of glycerol, methanol, and fatty acids, with ME as a percent of GE averaging 85.4% .
Although crude glycerol can be added to animal feed, excess glycerol in the animal diet may affect normal physiological metabolism. A few manuscripts have been published that focused on the levels of crude glycerol fed and the performance of crude glycerol in animal feeds. The improvement of daily gains by pigs depended on the actual intake of glycerol during the growing period but not on the finishing period. The dietary treatments had no significant effects on meat quality . When crude glycerol was added to the diets of weaned pigs, at levels up to 10%, the feed performance was enhanced . Up to 9% crude glycerol could be added to the diets of lactating sows with performances similar to sows fed standard corn-soybean meal control diets . No detrimental effects, with respect to egg performance, egg quality, nutrient retention, and metabolizable energy, were found when crude glycerol was incorporated at a level of 6% in the diet of laying hens . In broiler diets, increasing the intake level of crude glycerol increased feed conversion ratio but did not affect growth performance and nutrient digestibility . Crude glycerol was used effectively at levels of 2.5 or 5%. But the use of 10% crude glycerol resulted in poor feed flow. The influences of levels and quality of crude glycerol on pellet quality needs further study .
Crude glycerol in ruminant diets
Crude glycerol, added at up to 15% dry matter in the diets of finishing lambs, could improve feedlot performance, especially during the first 14 d, and had no associated effect on carcass characteristics . Compared to medium-quality hay, diets for meat goats with up to 5% crude glycerol proved to be beneficial . In addition, the inclusion of purified glycerol at up to 15% of the dry matter ration of lactating dairy cows was possible, without deleterious effects on feed intake, milk production, and yield . When crude glycerol was added at levels of 8% or less, based on dry matter in cattle finishing diets, it improved weight gain and feed efficiency . Apart from the above mentioned investigations, a patent described approaches for using or incorporating crude glycerol into animal feeds as well as feeding recommendations .
In all, the use of crude glycerol as an animal feed component has great potential for replacing corn in diets, and is gaining increasing attention. However, one must be aware of the presence of potential hazardous impurities in crude glycerol from biodiesel. For example, residual levels of potassium may result in wet litter or imbalances in dietary electrolyte balance in broilers . The levels of methanol must be minimized because of its toxicity. More attention should be paid to the crude glycerol from small scale biodiesel facilities that use simple batch distillation or evaporation techniques.
Feedstocks for chemicals
Chemicals produced via biological conversions
1, 3-propanediol
The anaerobic fermentative production 1, 3-propanediol is the most promising option for biological conversion of glycerol. Mu et al. demonstrated that crude glycerol could be used directly for the production of 1, 3-propanediol in fed-batch cultures of Klebsiella pneumoniae. The differences between the final 1, 3-propanediol concentrations were small for crude glycerol from the methanolysis of soybean oil by alkali- (51.3 g/L) and lipase-catalysis (53 g/L). This implied that the composition of crude glycerol had little effect on the biological conversion and a low fermentation cost could be expected. Further, the production of 1,3-propanediol by K.pneumoniae was optimized using response surface methodology. The maximum yield of 1, 3-propanediol was 13.8 g/L. More recently, the production of 1, 3-propanediol, from crude glycerol from Jatropha biodiesel by K.pneumoniae ATCC 15380, was optimized. The obtained 1, 3-propanediol yield, purity and recovery were 56 g/L, 99.7% and 34%, respectively. Additionally, an incorporated bioprocess that combined biodiesel production by lipase with microbial production of 1, 3-propanediol by K.pneumoniae was developed in a hollow fiber membrane. The bioprocess avoided glycerol inhibition on lipase and reduced the production cost.
Clostridium butyricum also could be used to produce 1, 3-propanediol from crude glycerol. For example, C. butyricum VPI 3266 was able to produce 1, 3-propanediol from crude glycerol on a synthetic medium. Trivial differences were found between commercial glycerol and crude glycerol. C.butyricum strain F2b and C.butyricum VPI 1718 potentially could convert crude glycerol to 1, 3-propanediol . In order to avoid the isolation of 1,3-propanediol from crude glycerol fermentation media, a one vessel bio- and chemo-catalytic process was developed to convert crude glycerol to secondary amines directly in a biphasic system without intermediate separation of 1, 3-propanediol . Additionally, Chatzifragkou et al. studied the effects of different impurities in crude glycerol on 1, 3-propanediol production by C.butyricum. The double bond from long-chain fatty acids or methyl esters might influence the growth performance of the microorganism, methanol did not affect the microbial conversion and the presence of NaCl had certain effect during a continuous process but not a batch process.
Citric acid
A few reports are available on the use of crude glycerol for citric acid biosynthesis. The production of citric acid from crude glycerol by Yarrowia lipolytica ACA-DC 50109 was not only similar to that obtained from sugar-based conventional media but also single-cell oil and citric acid were produced simultaneously . When a fed-batch fermentation by acetate-negative mutants of Y. lipolytica Wratislavia AWG7 strain was used to ferment crude glycerol, the final concentration of citric acid was 131.5 g/L, similar to that obtained from pure glycerol (139 g/L). On the other hand, when Y.lipolyticaWratislavia K1 was used, a lower concentration of citric acid (about 87-89 g/L) and a high concentration of erythritol (up to 47 g/L) were obtained . It was in line with the results shown by Rymowicz et al. Further, Y. lipolytica Wratislavia K1 proved to be superior to other strains by producing erythritol and not citric acid from crude glycerol under optimal conversion conditions, which may be a valuable development . Y.lipolytica LGAM S (7)1 also showed potential for converting crude glycerol to citric acid . More recently, it was reported that Y. lipolytica N15 could produce citric acid in high amounts, specifically, up to 98 g/L of citric acid and 71 g/L of citric acid were produced from pure glycerol medium and crude glycerol medium, respectively .
Hydrogen and other lower molecule fuels
The bacterium Rhodopseudomonas palustris was capable of photofermentative conversion of crude glycerol to hydrogen. Nearly equal productions were obtained from crude glycerol and pure glycerol. Up to 6 moles H2 per mole glycerol were obtained, which was 75% of theoretical. Both rates and yields of hydrogen production could be modified by changing the concentration of added nitrogen. However, some technical obstacles, such as enhancing the efficiency of light utilization by the organisms and developing effective photobioreactors, still need to be solved during development of a practical process . When Enterobacter aerogenes HU-101 was employed, hydrogen and ethanol were produced at high yields and with high production rates. But the crude glycerol should be diluted with a synthetic medium in order to increase the rate of glycerol utilization . For maximizing hydrogen production, Jitrwung and Yargeau optimized some media compositions of E. aerogenes ATCC 35029 fermented crude glycerol process. More recently, it was reported that K.pneumoniae mutant strain and nonpathogenic Kluyvera cryocrescens S26 were promising for producing ethanol from crude glycerol. In addition, crude glycerol, as a co-substrate, could be used to enhance hydrogen and especially methane production during the anaerobic treatment of different feedstocks including the organic fraction of municipal solid wastes, sewage sludge and slaughterhouse wastes.
Poly (hydroxyalkanoates)
Poly(hydroxyalkanoates) (PHA) represent a complex class of naturally occurring bacterial polyesters and have been recognized as good substitutes for non-biodegradable petrochemically produced polymers. Ashby et al. reported that crude glycerol could be used to produce PHA polymer. PHB is the most-studied example of biodegradable polyesters belonging to the group of PHA. The study of the feasibility of using crude glycerol for PHB production, with Paracoccus denitrificans and Cupriavidus necator JMP 134, showed that the resulting polymers were very similar to those obtained from glucose. But the PHB production decreased significantly when NaCl-contaminated crude glycerol was used. The authors suggested that the harmful effect of the NaCl-contaminant could be reduced by mixing crude glycerol from different manufacturers. Further, a process based on the Cupriavidus necator DSM 545 fermentation of crude glycerol was designed for the large-scale production of PHB. However, sodium still hindered the cell growth . Zobellella denitrificans MW1 could utilize crude glycerol for growth and PHB production to high concentration, especially in the presence of NaCl. Therefore, it was recommended as an attractive option for large-scale production of PHB with crude glycerol .
Additionally, when mixed microbial consortia (MMC) was used for PHA production from crude glycerol, it was found that methanol in the crude glycerol was transformed to PHB by MMC. Further, it was estimated that a 10 million gallon per year biodiesel plant would have the potential of producing 20.9 ton PHB. More recent report showed that Pseudomonas oleovorans NRRL B-14682 could also be used for PHB production from crude glycerol.
Docosahexaenoic acid
A series of papers on the production of docosahexaenoic acid (DHA)-rich algae were published, using crude glycerol, by fermentation of the alga Schizochytrium limacinum. For supporting alga growth and DHA production, 75-100 g/L concentration of crude glycerol was recommended as the optimal range. The algal DHA yield was influenced significantly by temperature and ammonium acetate concentration. The optimal amounts for temperature and ammonium acetate were 19.2°C and 1.0 g/L, respectively. The highest DHA yield obtained was 4.91 g/L under the optimized culture conditions. Different sources of crude glycerol did not result in significant variations in algal biomass compositions. The resulting algae had a similar content of DHA and a comparable nutritional profile to commercial algal biomass. That proposed good potential for using crude glycerol-derived algae in omega-3-fortified foods or feeds. Further, DHA-containing algae have been developed as replacements for fish oil for omega-3 fatty acids. Crude glycerol was used to produce fungal biomass that served as eicosapentaenoic acid (EPA)-fortified foods or feeds through fungal fermentation with fungus Pythium irregulare. Growing in medium containing 30 g/L crude glycerol and 1.0 g/L yeast extract, the EPA yield and productivity could reach 90 mg/L and 14.9 mg/L per day, respectively. The resulting EPA content was low compared to microalgae for EPA. Optimizing culture conditions and developing high cell density culture techniques are imperative in future work. Recently, it was reported that continuous culture was an effective approach for studying the growth kinetics and behaviors of the algae on crude glycerol.
Lipids
As the sole carbon source, crude glycerol could be used to produce lipids which might be a sustainable biodiesel feedstock. For example, crude glycerol could be used for culturing Schizochytrium limacinum SR21 and Cryptococcus curvatus. S. limacinum algal growth and lipid production were affected by the concentrations of glycerol. Higher concentrations of glycerol had negative effects on cell growth. For batch culturing of crude glycerol derived from yellow grease, the optimal glycerol concentrations for untreated and treated crude glycerol were 25 and 35 g/L, respectively. With 35 g/L, the obtained highest cellular lipid content was 73.3%. Methanol remaining in crude glycerol could harm S. limacinum SR21 growth. For C. curvatus yeast, fed-batch was a better process than batch for lipid production. Culturing for 12 days, the lipid content from one-stage fed-batch operation and two-stage fed-batch process were 44.2% and 52%, respectively. Methanol did not have significant inhibitory effect on cell growth. The produced lipid had high concentration of monounsaturated fatty acid and was good biodiesel feedstock.
Further, Saenge et al. presented that oleaginous red yeast Rhodotorula glutinis TISTR 5159, cultured on crude glycerol, produced lipids and carotenoids. The addition of ammonium sulfate and Tween 20 increased the accumulation of lipids and carotenoids. When fed-batch fermentation was employed, the highest lipid content, lipid yield and carotenoids production were 10.05 g/L, 60.7% and 6.10 g/L, respectively. Chlorella protothecoides also converted crude glycerol to lipids. The lipids yield was 0.31 g lipids/g substrate. Similarly, with C. protothecoides and crude glycerol (62% purity), Chen and Walker demonstrated that the maximum lipid productivity of 3 g/L per day was obtained in a fed-batch operation, which was higher than that produced by batch process. Additionally, Chatzifragkou et al. studied the potential of fifteen eukaryotic microorganisms to convert crude glycerol to metabolic products. The results showed that yeast accumulated limited lipids (up to 22 wt.%, wt/wt, in the case of Rhodotorula sp.), while fungi accumulated higher amounts of lipids in their mycelia (ranging between 18.1 and 42.6%, wt/wt, of dry biomass).
Other chemicals
Beyond the chemicals mentioned above, several other processes for producing useful chemicals from crude glycerol via biotransformations have been developed. A continuous cultivation process and a recently isolated bacterium Basfia succiniciproducens DD1 were identified for succinic acid production. The process was characterized as having great process stability, attractive production cost, and impossible pathogenicity of the production strain, but the final production strain needs to be examined further for commercial succinic acid production. Via simulation method, Vlysidis et al. showed that the succinic acid co-production from crude glycerol, for a 20 years biodiesel plant, would improve the profit of the overall biorefinery by 60%.
Further, crude glycerol, as the sole carbon source, had the potential of producing phytase in industrial scale in high cell density fermentations with recombinant Pichia pastoris possessing a pGAP-based constitutive expression vector and producing butanol with Clostridium pasteurianum. The highest yield of butanol was 0.30 g/g, which was significantly higher than the 0.15-0.20 g/g butanol yield typically obtained during the fermentation of glucose using Clostridium acetobutylicum. However, further understanding and optimizing of the process are still needed. It remained unclear what impact the impurities in crude glycerol would have on the solvent formation. Similarly, crude glycerol could be used in a bioprocess with P. pastoris without any purification. Canola oil-derived crude glycerol was the most favorable carbon source and showed great potential for the production of additional value-added products such as the recombinant human erythropoietin and cell growth. Crude glycerol also could be economic carbon and nutrient sources for bacterial cellulose (BC) production. The BC amount obtained was about 0.1 g/L after 96 h incubation. The addition of other nutrient sources (yeast extract, nitrogen and phosphate) to crude glycerol culture media increased the BC production by ~200%.
Additionally, Gluconobacter sp. NBRC3259 could be used to produce glyceric acid from crude glycerol with an activated charcoal pretreatment. 49.5 g/L of glyceric acid and 28.2 g/L dihydroxyacetone were produced from 174 g/L of glycerol . When Staphylococcus caseolyticus EX17 was employed, crude glycerol could be used for solvent tolerant lipase production . More recently, it was reported that Ustilago maydis was a good biocatalyst for converting crude glycerol to glycolipid-type biosurfactants and other useful products . Fungal protein, Rhizopus microsporus var. oligosporus, production on crude glycerol was another potential use of crude glycerol. The obtained fungal biomass contained high amounts of threonine and could be co-fed with commercial sources. But feeding formulation need be further studied .
Chemicals produced through conventional catalytic conversions
Oxygenated chemicals
As fuel additive, the oxygenate synthesized compound, (2,2-dimethyl-1,3-dioxolan-4-yl) methyl acetate, could be produced from crude glycerol and used as a biodiesel additive. It could improve biodiesel viscosity and could meet the requirements established for diesel and biodiesel fuels by the American and European Standards (ASTM D6751 and EN 14214, respectively) for flash point and oxidation stability. This new compound could compete with other biodiesel additives . Further, acrolein is an important starting chemical for producing detergents, acrylic acid ester and super absorber polymers. Sereshki et al. reported a process involving adding liquid crude glycerol directly into a fluidized bed reactor, vaporizing it, and then reacting them to produce acrolein over a tungsten doped zirconia catalyst. In this process, glycerol evaporated in the fluidized bed reactor leaving behind salt crystals which were only loosely bound to the surface and could be separated from the catalyst using mechanical agitation. This process has the potential to reduce the accumulation of salt in the reactor.
Hydrogen or syngas
Crude glycerol was proven to be a viable alternative for producing hydrogen or syngas . Gasification was the main employed technique. Thermo-gravimetry coupled with FTIR spectroscopy analysis proved that the thermal decomposition mechanism of crude glycerol mainly involved four phases and CO2, H2, CH4, and CO were the main gas products . Gasification with in situ CO2 removal was effective and had high energy efficiency . Supercritical water gasification of crude glycerol was performed under uncatalyzed and alkaline catalyzed conditions. The reaction temperature determined the decomposition degree. When NaOH was employed as catalyst nearly 90 vol.% of the product gas was H2 and no char was produced . Additionally, co-gasification of crude glycerol and hardwood chips, in a downdraft gasifier, was another promising option for utilizing crude glycerol. The loading amount of crude glycerol had significant influence on CO and CH4 concentration, while having no effect on H2 and CO2 yield. The study suggested that the co-gasification could perform well in downdraft gasifiers with hardwood chips mixing with liquid crude glycerol up to 20 wt.% .
Other chemicals from conventional catalytic conversions
Addition to the previous mentioned chemicals produced from crude glycerol, several other chemicals from conventional catalytic conversion processes were reported. Monoglycerides could be produced via glycerolysis of triglyceride with crude glycerol. A two-step process, involving the removal of residual glycerol and crystallization, was employed for purification of the monoglycerides produced from glycerolysis. An approximate 99% purity monopalmitin was achieved . More recently, the glycerolysis of soybean oil, using crude glycerol, was investigated. The amount of inorganic alkaline catalyst in the crude glycerol affected the concentration of the produced monoglycerides. Crude glycerol containing lower content of inorganic catalyst (about 2.7 wt.% NaOH) produced the highest concentration of monoglycerides, which was about 42% .
Other uses
Beyond the aforementioned uses, a few other potential applications of crude glycerol have been reported. Crude glycerol was used as a high-boiling-point organic solvent to enhance enzymatic hydrolysis of lignocellulosic biomass during atmospheric autocatalytic organosolv pretreatment . Crude glycerol (without any purification) also could be used as a green solvent for organic reactions. Two representative reactions were base catalyzed aldol condensation and palladium catalyzed Heck carbon-carbon coupling . Functioning as an organic carbon source for the removal of nitrate in the wastewater denitrification process, the denitrification efficiency could be increased by 2.0-5.0 mg NO3-N L-1 per crude glycerol dose of 100 L 1. Additionally, crude glycerol could be used as a fuel for generating electricity from microbial fuel cells . Both co-hydrothermal pyrolysis and co-liquefaction of manure with crude glycerol could improve the production yield of bio-oil. However, for the co-liquefaction, too much addition of crude glycerol affected the carbon content and heat value of the bio-oil .
Conclusions
Effective utilization of crude glycerol is very crucial to the commercialization and further development of biodiesel production. In the long term, utilization of biomass-derived glycerol will not only contribute to reducing society's dependence on nonrenewable resources but also will promote the development of integrated biorefineries.
This review addresses the value-added opportunities for crude glycerol from biodiesel production, mainly as a feedstuff for animal feed and as feedstocks for chemicals. Promising results have been achieved by researchers, especially for non-ruminant animals such as pigs, laying hens, and broilers. However, there still are some considerations that need to be taken into account for broad-scale use of this biomass-derived chemical in animal feeds. First of all, the chemical composition of crude glycerol varies significantly with the methods and feedstocks used to produce biodiesel. Since different researchers have used different qualities of crude glycerol for their studies, animal producers need to pay attention when they decide to include crude glycerol as a component of animal feed rations. Secondly, to some extent the impurities in crude glycerol affect feed performance. Finally, the amount of crude glycerol included in feed formulations needs to be considered. The establishment of a crude glycerol feed specification is recommended. The resulting "standard" crude glycerol would have a higher value simply because it would be consistent from all producers.
Conventional catalysis and biotransformation are the two main routes available for converting crude glycerol to a variety of value-added chemicals. In more recent years, there have been extensive studies, and some encouraging results, on processes for crude glycerol conversion. For example, the productions of 1,3-propanediol, citric acid, poly (hydroxyalkanoates), butanol, hydrogen, docosahexaenoic acid-rich algae, monoglycerides, lipids and syngas from crude glycerol are promising. However, many of the technologies offered still need further development to make them cost-effective and operationally feasible for incorporation into biorefineries.
Impurities in crude glycerol can greatly influence the conversion of glycerol into other products. In some biological conversion processes, pollutants in crude glycerol, inhibit cell and fungal growth and result in lower production rates and product yields (compared with pure or commercial glycerol under the same culture conditions). On the other hand, for conventional catalytic conversions, impurities poison the catalysts, increasing char production and influencing product yield. Many of the technologies need to be more fully understood and optimized, such as optimizing reaction parameters, production yields, and fermentation conditions, developing mutant strains and efficient bioreactors for stable cultures, and improving the activity and selectivity of catalysts. Additionally, cell growth has been shown to be inhibited during fermentation when the initial crude glycerol concentration was high. Conversely, higher crude glycerol concentrations are necessary for improving production efficiency.
In brief, it has been shown that biological conversions of crude glycerol can produce higher value chemicals. However, it is impossible for the current state of the technologies to convert glycerol at rates necessary to prevent a large accumulation of glycerol. Both improved biological and conventional techniques will be needed in future biodiesel plants. Other promising uses, starting with crude glycerol, also have been disclosed. Although hopeful results have been achieved, there are still aspects that need to be improved.
In summary, in recent years, scores of utilization opportunities of crude glycerol have been presented and promising results have been achieved. However, it is imperative to point out that there are still more technical hurdles to jump for developing practical processes to directly utilize crude glycerol from biodiesel production on a large scale. Quick overviews of what has been investigated, with respect to potential chemicals from biological and conventional catalytic conversions of crude glycerol, are summarized in Tables and , respectively.
Table 1
Biological conversions of crude glycerol to chemicals
Product | Pathway | Product productivity | Reference |
|---|---|---|---|
1, 3-propanediol | Fed-batch cultures of Klebsiella pneumoniaestrain | 1.7 g/L/h | |
Maximum 1,3-propanediol production from K. pneumonia | 13.8 g/L | ||
Optimize 1,3-propanediol production from K. pneumoniae ATCC 15380 | 56 g/L | ||
Integrated bioprocess combining biodiesel production by lipase with microbial production of 1, 3-propanediol by K. pneumoniae strain | 1.7 g/L/h | ||
Clostridium butyricum strain VPI 3266 on a synthetic medium | 0.60 mol/mol glycerol | ||
C. butyricum strain F2b (process modelling) | NAa | ||
C. butyricum VPI 1718; fed-batch operation under non-sterile culture conditions | 67.9 g/L | ||
One vessel bio- and chemocatalytic process; in a biphasic system without intermediate separation of 1, 3-propanediol; C. butyricumDSM10703 | 134 m mol/L | ||
Citric acid | Y. lipolytica strain ACA-DC 50109 (process modelling) | NAa | |
Acetate Mutants of Y. lipolytica Wratislavia AWG7 strain; Fed-batch operation | 139 g/L | ||
Yarrowia lipolytica strain LGAM S (7)1 | 35 g/L | ||
Y. lipolytica N15 | 71 g/L | ||
Erythritol | Fed-batch cultures of Y. lipolyticaWratislavia K1 | 1 g/L/h | |
Hydrogen | Photofermentative conversion process; Rhodopseudomonas palustris strain | 6 mol/mol glycerol | |
Enterobacter aerogenes strain HU-101; continuous culture; porous ceramics as a support material to fix cells | 63 mmol/L/h | ||
Optimize some media compositions of E. aerogenes ATCC 35029 | 0.85 mol/mol glycerol | ||
Anaerobic treatment process; crude glycerol was a co-substrate | 2.9 mmol/g glycerol | ||
Poly (hydroxyalkanoates) (PHAs) | Pseudomonas oleovorans NRRL B-14682 and P. corrugata 388 grew and synthesized PHB and mcl-PHA, respectively | NAa | |
Producing PHB; Paracoccus denitrificansand Cupriavidus necator JMP 134 strains | 48% | ||
Producing PHB; Cupriavidus necator strain DSM 545 | 50% | ||
Producing PHB; Zobellella denitrificansMW1; fed-batch cultivation | 66.9% ± 7.6% | ||
Producing PHB; Mixed microbial consortia (MMC) | > 50% | ||
Pseudomonas oleovorans NRRL B-14682; batch culture | 30% | ||
Phytase | High cell density fermentations, recombinant Pichia pastoris | 1125 U/mL | |
Lipase | Staphylococcus caseolyticus EX17 | 127.3 U/L | |
Succinic acid | Basfia succiniciproducens DD1; continuous cultivation process | 1.02 g/g glycerol | |
Docosahexaenoic acid-rich algae | Fermentation of the alga, Schizochytrium limacinum strain; continuous culture | 0.52 g/L-day | |
Eicosapentaenoic acid | Fungus Pythium irregulare | 90 mg/L | |
Lipid | Schizochytrium limacinum SR21; batch culture | 73.3% | |
Cryptococcus curvatus; two-stage fed-batch process | 52% | ||
Oleaginous red yeast Rhodotorula glutinisTISTR 5159; fed-batch fermentation | 60.7% | ||
Chlorella protothecoides | 3 g/L per day | ||
Fungi | 42.6% | ||
Recombinant human erythropoietin etc. | Pichia pastoris medium | 31 mg/L | |
Ethanol | Nonpathogenic Kluyvera cryocrescens S26; batch fermentation | 27 g/L | |
Klebsiella pneumoniae mutant strain (GEM167) | 21.5 g/L | ||
Methane | Anaerobic digestion | 0.306 m3/kg glycerol | |
Butanol | Clostridium pasteurianum (ATCC®6013™) | 0.30 g/g glycerol | |
Fungal protein | Rhizopus microsporus var. oligosporus | 0.83 ± 0.02 g/g glycerol |
a represents data are not available
Table 2
Conventional catalytic conversions of crude glycerol to chemicals
Product | Pathway | Product productivity | Reference |
|---|---|---|---|
(2,2-dimethyl-1,3-dioxolan-4-yl) methyl acetate | Oxygenate synthesized compound | NAa | |
Acrolein | Fuidized bed, tungsten doped zirconia catalyst | 21% | |
Monoglyceride | Two-step process, purification of the monoglyceride produced from glycerolysis of palm stearin | ~99% purity | |
Glycerolysis of soybean oil | ~42% | ||
Gaseous products | Steam reforming process; platinum alumina as catalyst | NAa | |
Thermal decomposition of crude glycerol by pyrolysis | NAa | ||
Steam gasification with in situ CO2removal | 88 vol.% H2purity | ||
Hydrothermal reforming of crude glycerol | ~90 vol.% H2purity | ||
Co-gasification of crude glycerol and hardwood chips | NAa |
a represents data are not available