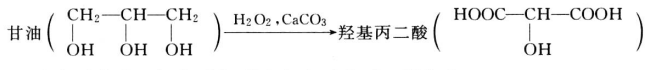
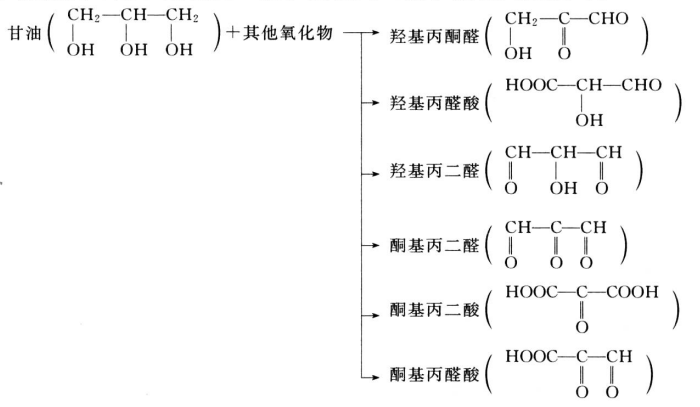
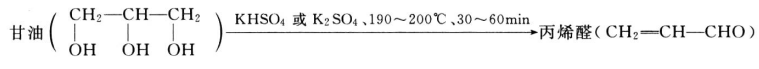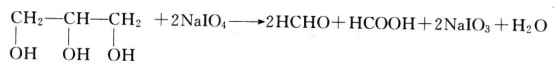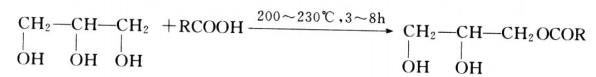The Glycerin market is expected to grow at a moderate rate during the forecast period, 2018 to 2023. Asia-Pacific region is estimated to lead the market owing to its increasing usage in personal care and pharmaceutical applications. Pharmaceutical application is expected to be the largest market.
- Increasing production and demand for biodiesel
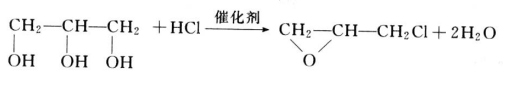
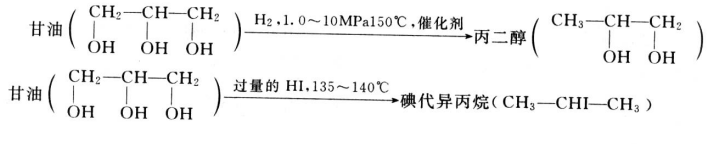
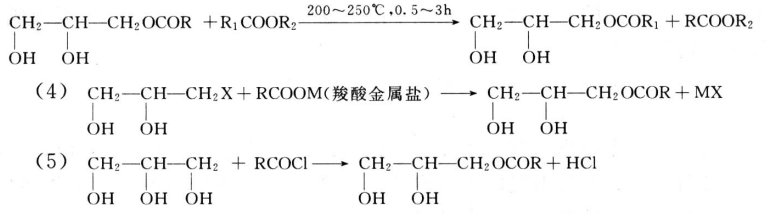
Glycerin is a by-product, produced while manufacturing biodiesel from animal fats, and vegetable oil. Rising demand for alternative fuel vehicles among consumers in European Union and North America has increased the consumption of biodiesel. According to European Automobile Manufacturer Association (ACEA), 852,933 alternative fuel vehicles were registered in the European Union in 2017, which is estimated to increase the demand for biodiesel during the forecast period. This increase in demand for bio-fuel, coupled with increasing concern related to global climate change, improving energy stability, and fluctuating crude oil prices is expected to increase the demand for biodiesel, which, in turn, is expected to increase the availability of glycerin during the forecast period.
- Pharmaceutical Application will continue to Dominate the Market
Pharmaceutical application will continue to dominate the market for glycerin during the forecast period. Glycerin is an important raw material used for manufacturing products such as creams, jellies, eye-drops, syrups, and lozenges among others due to its smoothing, moisturizing, and sweetening properties. Increase in spending on medication and rising use of new and expensive medicinal products due to increasing health concern has propelled the consumption of pharmaceutical products in the recent years. In addition, the growing medical tourism in countries, such as India, Mexico, Thailand, South Korea, Turkey, Malaysia, Costa Rica, Brazil, and Taiwan has increased the consumption of various pharmaceutical products. For instance, according to OECD, around 50 million tourist seek medical treatment annually, on the global level, which in turn has driven the demand for glycerin in pharmaceutical application.
- Asia-Pacific Region to lead the market
Asia-Pacific is projected to dominate the glycerin market, during the forecast period, owing to the growing demand from personal care application. Increase in consumer spending on mass and premium personal care products coupled with increasing population in countries, such as India, China, Japan, and South Korea has increased the consumption of glycerin. In addition, rise in cosmetics sales due to growing tourism industry in countries, such as Thailand, Vietnam, and Cambodia has increased the production of personal care products, which in turn is expected to drive the demand for glycerin for personal care application during the forecast period.