In the biodiesel reaction, oil is converted to biodiesel and glycerin in the presence of methanol and other chemicals. Glycerin is about 10 percent of the final product. When you drain off the glycerin, it contains the excess methanol not used by the reaction. Please use caution, because fumes from this mixture are very flammable and dangerous to your health.
Wash water is used to flush salts and impurities from the finished biodiesel. This water also contains unreacted oil and some methanol and biodiesel.
Glycerin and wash water are high-strength wastes and much more concentrated than the usual wastewater discharged to a sewer system. In addition, glycerin will gel at lower temperatures and can clog up plumbing. Never discharge glycerin or wash water to a septic system. This will overload the process and could lead to clogging of the laterals.
In large quantities, these wastes can damage the treatment process at a municipal wastewater plant, but the amounts of glycerin and wash water created by a small producer should not effect the operation of a public treatment works. Glycerin can gel and clog pipes, but it is water soluble. Therefore, if you flush the pipes with water as you pour out the glycerin, you may avoid gelling. Again, use caution around sources of ignition. You can put wash water down the drain without causing problems.
Because of the possibility of clogging pipes, it might be better to talk to the local treatment plant operators and bring the raw glycerin directly to the treatment facility.
Never put waste glycerin or wash water into a storm sewer or ditch. This would send the waste directly into a stream. The high biochemical oxygen demand from the glycerin will greatly lower oxygen levels in the stream and cause a fish kill. Direct discharge of these wastes to waters of the United States is a criminal violation!
When the household hazardous waste exemption applies, landfills can accept glycerin, but most landfills are not eager to accept a liquid waste.
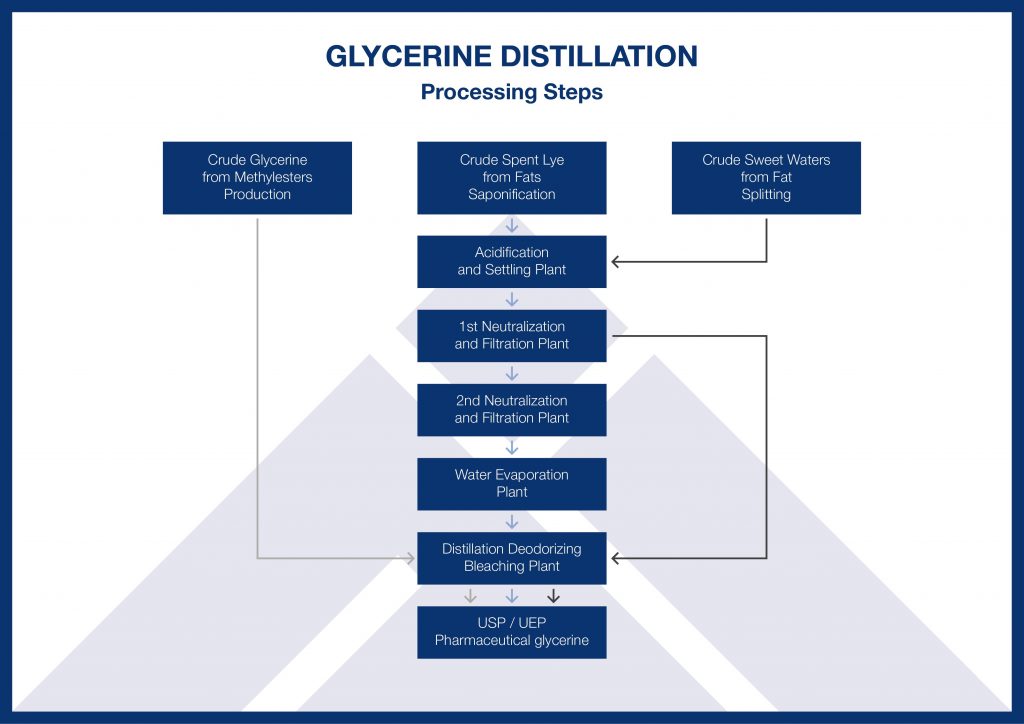
Industrial biodiesel operations often refine crude glycerin to create value-added products. This is beyond the capabilities of most non-commercial and home biodiesel producers, but some small-scale producers use the glycerin to make soap after removing the methanol.