The relative density of pure glycerin at 20°c is 1.6362, which is higher than water density. The density changes as the change of temperature. The temperature increases and the relative density decreases. The relative density of glycerin aqueous solution increases with the increase of glycerin content, so the purity of glycerin can be determined by relative density. The result is quite exact. So the method is widely used as the method of determination of glycerin concentration. The conversion relation between the relative density and the concentration of glycerin aqueous solution refers to table 2. 2. the relationship between the concentration of glycerol aqueous solution and the relative density at low temperature is shown in table 2.3.
Table 2.2 Conversion relationship between relative density and concentration of glycerin aqueous solution
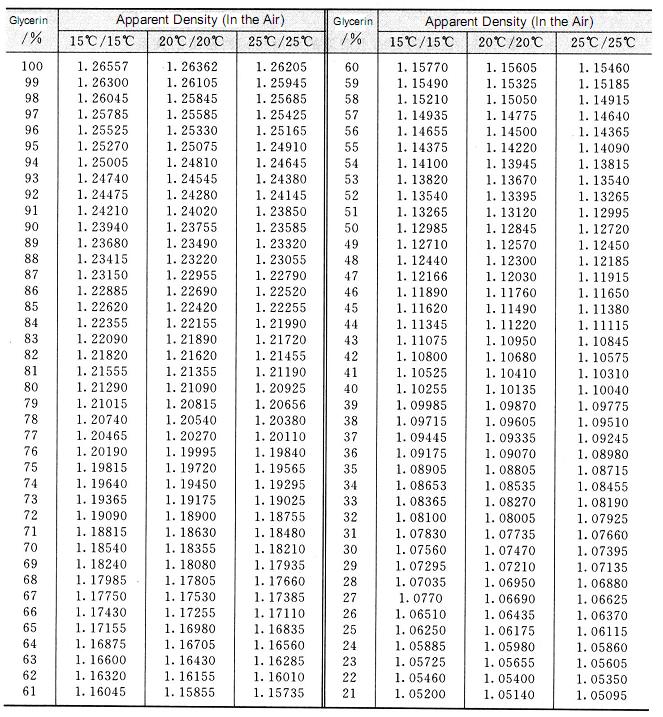
Continued Table
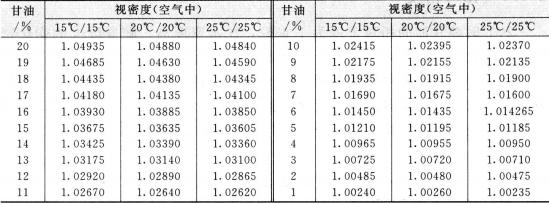
Table 2.3 The relationship between concentration and relative density of glycerol aqueous solution at low temperature
2.1.2 Glycerin’s Viscosity
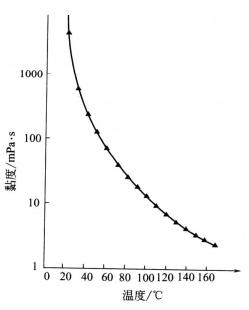
Glycerin’s viscosity is very high, which is a very prominent characteristic of glycerin. The viscosity varies with the temperature, the temperature increases, and the viscosity decreases (see figure 2.2). At a certain temperature, the viscosity of the oil-water solution increased with the increase of glycerin concentration (see Figure 2.3).
When there is moisture in the glycerol, the viscosity calculation formula is as follows:
Lg η=l. 9367-2. 700X 10³/T+0. 90X 10^6/T^2-0. 043c
In the formula, η is water containing c*% (mass) and the dynamic viscosity (freezing point = 273.16K) when the thermodynamic temperature is T.
In addition, glycerin’s viscosity increases rapidly with the increase of pressure, as well as its growth rate. If the Lg η/P and P mapping, it almost forms into a straight line relationship ( η=30°c and the viscosity of glycerin under Latm❶, p = pressure)
2.2 The relationship between viscosity and temperature of pure glycerin
2.3 The relationship between glycerin concentration and viscosity
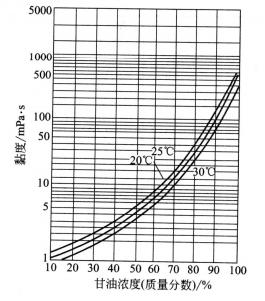
Electrolyte in pure glycerin and glycerin aqueous solution, usually increases the viscosity of glycerol, but there are also exceptions, such as ammonium chloride, ammonium bromide, ammonium iodide, rubidium chloride and bromide, nitrate etc. The higher concentration of these salts, the higher the concentration of glycerin, the lower viscosity.
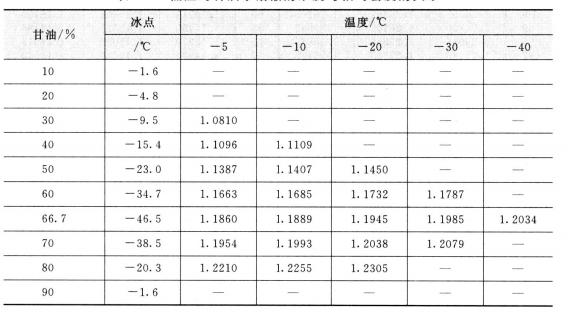
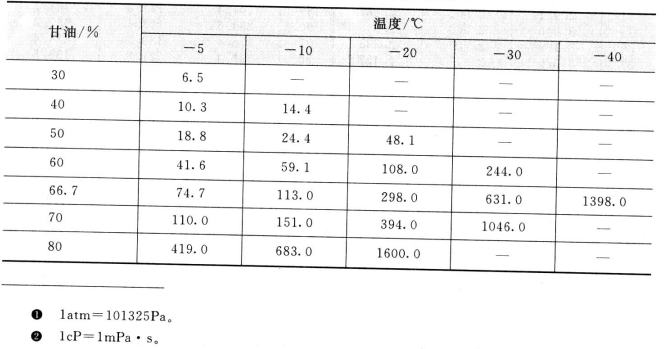
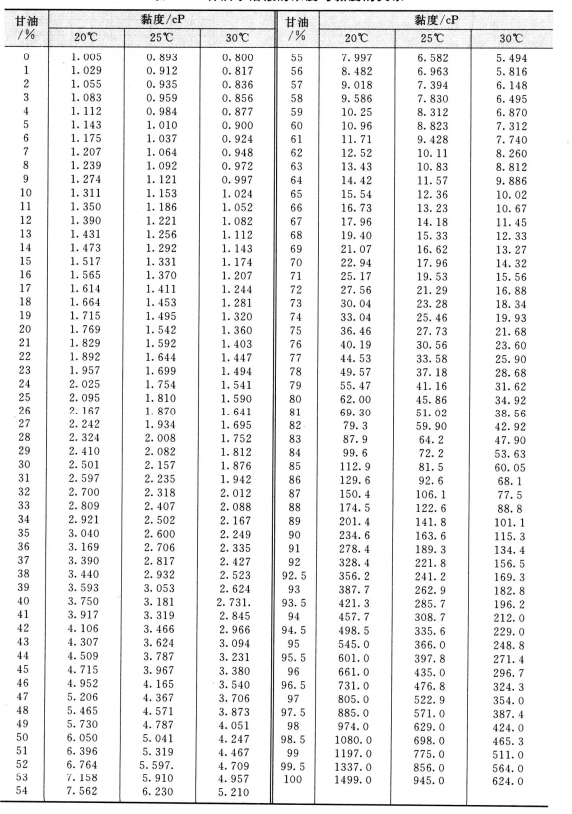
The viscosity of glycerin aqueous solution at low temperature (CP) and the concentration and viscosity of glycerol aqueous solution are shown in table 2. 4, Table 2.5.
2.4 Viscosity of glycerol water concentration at low temperature/cP
2.5 The relationship between concentration and viscosity of glycerin aqueous solution
No comments:
Post a Comment