3 Terms and definitions
The following terms and definitions apply to this standard.
3.1
Color and lustre unit for Hazen
The color of platinum in each liter solution (chloro platinic acid) 1mg and cobalt chloride hexahydrate 2mg.
3.2
Density at 20℃
The quality of material of unit volume at 20℃, which is expressed in grams per milliliter.
4 Sampling method for barrel glycerin
4.1 General rules
This method is suitable for
refining glycerol without solids or suspended solids in the barrel. It is equally applicable to barrel
Refined Glycerin, which can be restored to the original state after being frozen and heated.
The samples used for laboratory determination are prepared and stored according to this method.
4.2 Principle
Sampling tube is inserted from the plug hole to the bottom of the barrel. Samples are taken from the whole depth of the barrel, and they are taken in equal quantities for per sample barrel. All samples of the same batch were mixed evenly and divided into laboratory samples with the required number.
4.3 Instrument
4.3.1 Sampling Tube
As shown in figure 1, It consists of two stainless steel or other chemical resistant cylinders, and the inner cylinder is tightly matched with the outer cylinder. Each of the two cylinders has two rows of interlaced longitudinal grooves, the width of the groove accounts for a quarter of the circumference of the cylinder, the length of the groove is quarterly distributed over the length of the cylinder. The grooves on the inner and outer cylinder can coincide or seal precisely by rotating the handle of the inner cylinder. With a pointer indicating the position of the ruler on the outer cylinder indicates the relative position of the grooves on the inner cylinder and the outer cylinder. In the “filling” position, the inner and outer grooves form two rows of interlaced and intermittent openings, so that samples of all depths in the barrel enter the sampling tube at the same time.
The inner and outer barrel bottoms are drilled with holes. When the pointer is in the “empty” position, the bottom holes coincide to form an opening, while the longitudinal groove remains sealed.
The length of the sampling tube should be proportional to the depth of the material to be sampled, and its effective volume should be about 0.1% of the volume of the barrel.
4.3.2 Wiping plug
Match with plug hole for sampling barrel.
4.3.3 Cylindrical collector
It is made of the same material as the sampling tube, but preferably glass. With a sealing cover and a volume of about 1.5L, the cylindrical collector is suitable for each ton of products to be sampled.
4.3.4 Sample bottle
It has a frosted glass stopper or a glass bottle with a polyethylene gasket cap, the volume of which is just the size of the prepared laboratory sample.
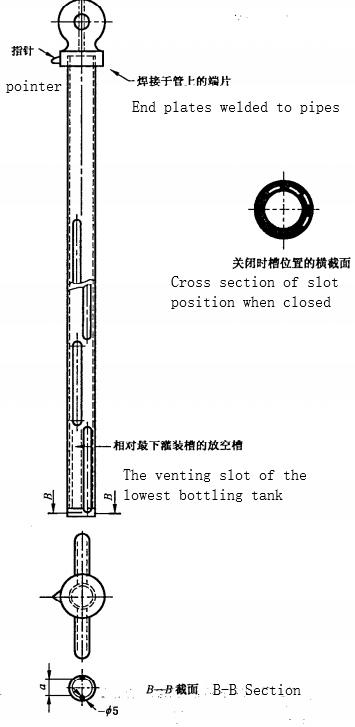
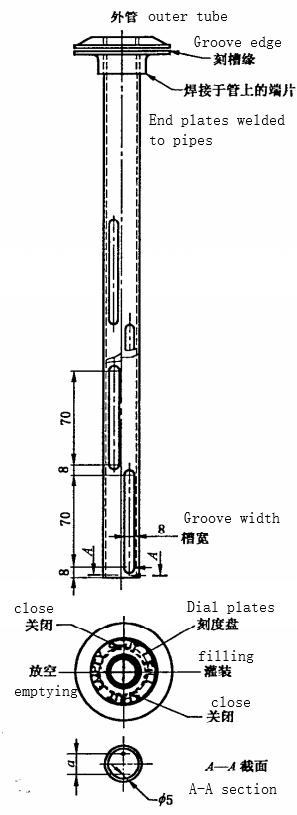
Figure 1 Glycerin Sampling Tube (Unit:mm)
4.4 Program
4.4.1 Preventive measure
Because glycerol is highly absorbent, the following precautions should be taken in sampling, analyzing and storing samples to avoid moisture
4.4.1.1 Containers used for mixing and storing samples shall be sealed, and operating space for canning and taking samples shall be kept sealed.
4.4.1.2 The containers should be shielded as far as possible during sampling, especially for rain and other accidental pollution.
4.4.1.3 All instruments and containers should be clean and dry when used.
4.4.1.4 The laboratory samples obtained from the mixed sample should be fully filled with sample bottles.
4.4.2 Sample preparation
The sampling tube (4.3.1) with the groove closed is inserted into the bottom of the barrel through a wipe plug (4.3.2), and the pointer is rotated to the “filling” position by rotating the handle. The longitudinal groove is opened. After the sampling tube is filled, the groove is closed, the sampling tube is extracted, and the outer wall of the tube is cleaned with the aid of a wipe plug.
Insert the glycerin-filled sampling tube into the collector (4.3.3), rotate the handle to the “empty” position and empty the sampling tube. The same amount of sample is taken from each barrel at the same time, so that the total amount is greater than the requirement. The space of the two emptying operation should keep the collector closed.
Close the sample container, lie down and roll, and quickly mix all the samples. Immediately remove 500 g (or other required) of the sample and place it in a sample bottle (4.3.4) so that the same number of laboratory samples are prepared. Cover tightly the bottle stopper or the sealing cover and seal it with sealing wax (or glue). Paste the sample label, which includes the sample name, batch number, specification, date of sampling and signature of the sample person.
If the glycerol in the sample barrel has been frozen, it should be softened and heated first and the barrel should be rolled backwards so that the glycerol can be defrosted and mixed before sampling according to the above.
5 Determination of transparency
5.1 Instrument Commonly used laboratory apparatus
5.1.1 Nano colorimetric tube, 50ml.
5.1.2 Milky white lighting
5.2 Program
The glycerol sample was mixed evenly and degassed by vacuum or ultrasonic wave. 50ML was measured, and in a nano colorimetric tube. The sample was observed by a milky white lamp at room temperature. The sample was then placed in front of a white screen to observe the reflection light. If there is no turbidity, that is transparent.
6 Determination of odour
Put a little
glycerin on the back of your hand and smell it. If there is only a special smell of glycerol and no other peculiar smell, that is, no bad smell.
7 Determination of colour and lustre
7.1 Principle
The color of the experiment was compared with that of the standard platinum-cobalt colorimetric solution, and the result was expressed in Hazen unit.
7.2 Reagent
Unless otherwise specified, only distilled or deionized water, or water of comparable purity, identified as analytical purity, is used in the analysis. Note: all experiments applicable to this standard.
7.2.1 CoCl2·6H2O
7.2.2 K2PtCl6
7.2.3 The density of hydrochloric acid is about 1.19g/ml.
7.3 Instrument
Commonly used laboratory apparatus.
7.3.1 Spectrophotometer, which wavelength ranges from 420nm to 800nm.
7.3.2 Nano colorimetric tube, 50ml or 100ml. There is a marking mark at 100mm above bottom.
7.3.3 Colorimetric tube holder, usually colorimetric tube bracket is white, it is best to install fluorescent lighting, to improve the effect of color observation.
7.4 Program
7.4.1 Preparation of standard colorimetric reserve solution
In a bottler with capacity of 100ml, cobalt chloride hexahydrate (7.2.1) 1g and potassium chloroplatinate (7.2.2) 1.245g were dissolved, hydrochloric acid (7.2.3) solution 100ml was
added, and water was diluted to the scale, and the mixture was uniform. The standard colorimetric reserve solution was obtained.
The standard colorimetric reserve solution is checked by spectrophotometer (7.3.1) with a 1 cm colorimetric cell at the following wavelengths. The range of absorbance is shown inTable 1.
Table 1 Absorbance range of standard colorimetric liquid storage
| Table 1 Absorbance range of standard colorimetric liquid storage |
| wavelength/nm | absorbance |
| 430 | 0.110-0.120 |
| 455 | 0.130-0.145 |
| 480 | 0.105-0.120 |
| 510 | 0.055-0.065 |
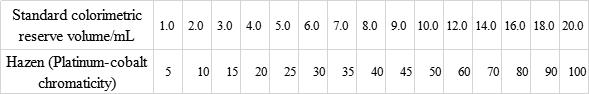
7.4..2 Preparation of platinum cobalt standard colorimetric solution
In 15 pcs 100ml volumetric flasks, add a standard colorimetric reserve (7.4.1) of the volume shown in Table 2, dilute it with distilled water, and shake well.
7.4.3 Storage of platinum cobalt standard colorimetric solution
Standard colorimetric reserve solution (7.4.1) and standard colorimetric solution (7.4.2) should be placed in stoppered brown glass bottles in the dark.
Standard colorimetric reserve liquids should be stored within the absorbance range shown in 7.4.1 before use. Otherwise, they should be reconfigured.
The standard colorimetric solution can be kept for one month, but it is better to use fresh configuration.
7.4.4 Determination
Samples are injected into a Nessler colorimetric tube (7.3.2) to the calibration line, different platinum-cobalt standard colorimetric solutions are injected into a series of Nessler colorimetric tubes to the calibration line, and placed on a colorimetric tube rack (7.3.3), each tube is coated with a black carton to avoid the influence of side light.
Compare the color of sample and platinum cobalt standard colorimetric solution. Colorimetry is directed at the white background illuminated by sunlight or fluorescent lamp, from top to bottom observation, select the closest color.
7.4.5 Result representation
The color of the specimen is expressed in the Hazen unit closest to the platinum cobalt standard colorimetric solution of the sample. If the color of the sample is between two platinum-cobalt standard colorimetric solutions, it is expressed in the Hazen unit of the platinum-cobalt standard colorimetric solution with a deeper color.
8.Determination of density at 20℃
8.1Principle
Measure the mass of the empty specific gravity bottle and the mass of water filled at 20℃ to determine the volume of the specific gravity bottle, and then measure the mass of the sample filled with the specific gravity bottle to calculate the density of glycerol at 20℃.
8.2 Instrument
Commonly used laboratory apparatus.
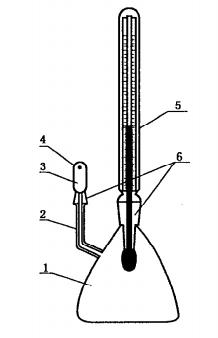
8.2.1 Specific gravity bottle, capacity 25ml-50ml, with thermometer, as shown in Figure 2.
1——Body of gravity bottle
2——Capillary tube
3——Cap
4——Air-bleed hole
5——Thermometer
6——Glass grinding joint
Figure 2 Gravity bottle
8.2.2 Thermostatic waterbath, It can maintain constant temperature at 20 ℃, accurate to 0.1 ℃, capacity over 1L.
8.2.3 Dryer, built in discolourant silica gel.
8.2.4 Analytical balance, accuracy 0.2mg
8.3 Program
8.3.1 Calibration of specific gravity bottle
Wash the specific gravity bottle carefully with potassium dichromate sulfuric acid lotion, distilled water, ethanol, acetone and so on. After drying, weigh it in a dryer (8.2.3) for 30 minutes and weigh it to 0.0002g. Boil and cool the distilled water at a temperature below 20 degrees to fill the specific gravity bottle to avoid bubbles. Insert the thermometer and put it in a constant temperature water bath at 20 degrees centigrade for 30 minutes. Quickly suck the overflowing water from the capillary with filter paper, cover the cap, remove the specific gravity bottle, carefully wipe the external surface of the specific gravity bottle, and place it in the dryer for 30 minutes to weigh. The apparent mass(m1) of water filled in a specific gravity bottle is calculated from mass difference.
8.3.2 Determination
Glycerol samples slightly lower than 20℃ were filled with the same clean and dry specific gravity bottle by 8.3.1 method for determination. Bubbles should be avoided when filling glycerol samples.
8.4 Result calculation
8.4.1 Computing method
The density(ρ20) of glycerol sample at 20 ℃ is g/mL, calculated by formula (1).

In formula (1)
m2 ——The apparent mass of the sample filled with 20 degree glycerol was g
A—— The quality of air filled in empty vase is g.
ρ20——Water density at 20℃, 0.9982g/mL
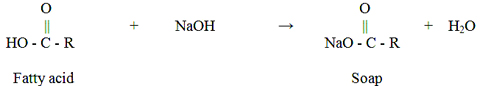
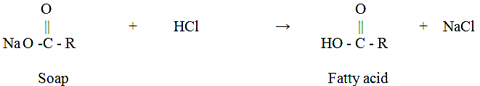









 It is in the ointments that diabetic patients are using externally. This substance used by those with constipation is also used as suppository.
It is in the ointments that diabetic patients are using externally. This substance used by those with constipation is also used as suppository.
 If it is applied on molds and similar difficult stains, it will allow the stain to come out of the stain as an obstacle to the fabrication process. It is intended for moisturizing purposes in personal care products such as skin care products, hair care products, mouthwashes, shaving creams and toothpaste.
It also has the following uses:
If it is applied on molds and similar difficult stains, it will allow the stain to come out of the stain as an obstacle to the fabrication process. It is intended for moisturizing purposes in personal care products such as skin care products, hair care products, mouthwashes, shaving creams and toothpaste.
It also has the following uses:

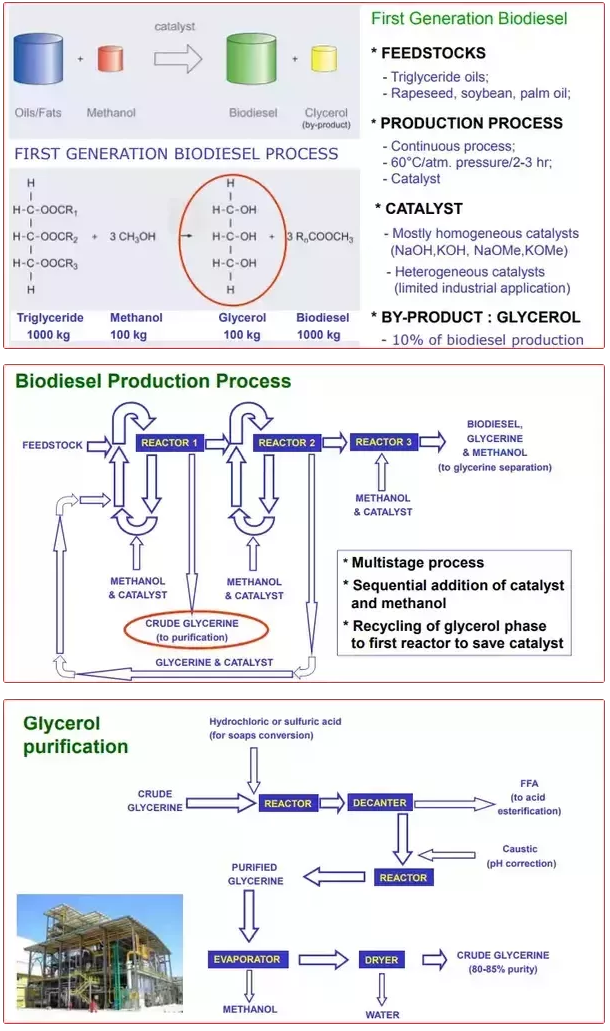
 Glycerine oil, which is used to get rid of dirt and inflammation in hair and skin care, has many benefits such as the one we have in this article, but at the same time there are various side effects. This ingredient, which is also included in cosmetic products, is almost used in every part of the body. Glycerin is a kind of liquid which is produced when the fatty substances saponify, sugary, odorless, burning, colorless and with certain consistency. It needs to be soap to get solid. If this material is applied to the skin, it will be possible to remove the cracks of the skin and soften it deeply. Although it is used medically to relieve ear and rheumatic pain, it can cause health problems if overdose is used.
If glycerine oil is used after the expiry date, all the benefits it has are left to various losses. On the other hand, if the cover of the consumed glycerin is left open, virus and bacteria formation is observed. Such a condition is particularly troublesome if glycerin is used by a person who has an infectious disease. However, there are various additives in this material. animal and vegetable glycerin is chemically more healthy than artificially produced glycerin.
Glycerine, which is also present in the cigarette, is irritated by respiratory tract as it is thrown out together with the cause of smoke. It is also known that glycerin, which is not produced as it should be, endangers every part of the body. However, blindness can occur even if this material comes into contact with the eye. It is possible that such a complication can arise if cosmetic products such as body and hand cream, shampoo and soap products are this product and this product is in contact with the skin. It is also possible to give skin allergic reactions if the dose is kept above normal, but not at an adequate level.
Glycerine oil, which is used to get rid of dirt and inflammation in hair and skin care, has many benefits such as the one we have in this article, but at the same time there are various side effects. This ingredient, which is also included in cosmetic products, is almost used in every part of the body. Glycerin is a kind of liquid which is produced when the fatty substances saponify, sugary, odorless, burning, colorless and with certain consistency. It needs to be soap to get solid. If this material is applied to the skin, it will be possible to remove the cracks of the skin and soften it deeply. Although it is used medically to relieve ear and rheumatic pain, it can cause health problems if overdose is used.
If glycerine oil is used after the expiry date, all the benefits it has are left to various losses. On the other hand, if the cover of the consumed glycerin is left open, virus and bacteria formation is observed. Such a condition is particularly troublesome if glycerin is used by a person who has an infectious disease. However, there are various additives in this material. animal and vegetable glycerin is chemically more healthy than artificially produced glycerin.
Glycerine, which is also present in the cigarette, is irritated by respiratory tract as it is thrown out together with the cause of smoke. It is also known that glycerin, which is not produced as it should be, endangers every part of the body. However, blindness can occur even if this material comes into contact with the eye. It is possible that such a complication can arise if cosmetic products such as body and hand cream, shampoo and soap products are this product and this product is in contact with the skin. It is also possible to give skin allergic reactions if the dose is kept above normal, but not at an adequate level.